Synthesis and In vitro Pharmacological Evaluation of Novel Variants of 4-Pyridyl Compound and Pyridyl Thiazole Substituents
Hemant Chikhale*, Sharma Lokesh P, Bhawsar Swati P, Suryawanshi Manjusha B, Patil Sachin V and Patil Amar A
Received Date: 2022-08-02 | Published Date: 2023-02-28Hemant Chikhale1*, Sharma Lokesh P2, Bhawsar Swati P2, Suryawanshi Manjusha B 3, Patil Sachin V3 and Patil Amar A3
1Department of Pharmaceutical Chemistry, Gosavi College of Pharmaceutical Education and Research, Maharashtra, India
2Department of Chemistry, HPT Arts and RYK Science College, Maharashtra, India
3Department of Microbiology, HPT Arts and RYK Science College, Maharashtra, India
- *Corresponding Author:
- Hemant Chikhale
Department of Pharmaceutical Chemistry,
Gosavi College of Pharmaceutical Education and Research,
Maharashtra,
India,
Tel: 9405211956;
Email: hemantch558@gmail.com
Received: August 02, 2022, Manuscript No. IPJSVP-23-16112; Editor assigned: August 05, 2022, PreQC No. IPJSVP-23-16112 (PQ); Reviewed: August 19, 2022, QC No. IPJSVP-23-16112; Revised: January 31, 2023, Manuscript No. IPJSVP-23-16112 (R); Published: February 28, 2023
Citation: Chikhale H, Lokesh PS, Swati PB, Manjusha BS, Sachin VP, et al. (2023) Synthesis and In vitro Pharmacological Evaluation of Novel Variants of 4-Pyridyl Compound and Pyridyl Thiazole Substituents. In Silico In Vitro Pharmacol Vol.9 No.1: 001.
Abstract
Background: Thiazoles are one of the most studied 5- membered aromatic heterocyclic. Many natural and synthetic thiazoles, as well as their derivatives, exhibited biological activity. Thiazole derivatives have antibacterial action against a variety of bacteria and diseases due to their unique characteristics. Because of its immense biological significance, scientists are working hard to develop novel biologically active thiazole compounds.
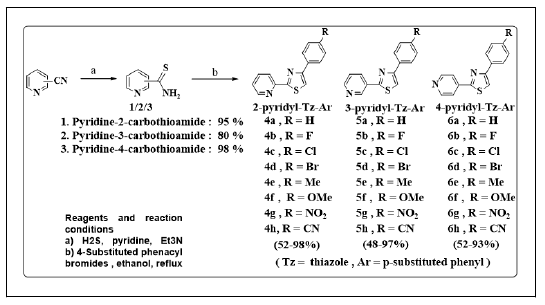
Method: In the present study the synthesis of some derivatives of the 2-pyridyl/3-pyridyl and 4-pyridyl substituent at C-2 and the aryl substituent at C-2 (scheme 1) design and the desired target molecules 2a-2g, 3a-3g and 4a-4g were synthesized via the classic Hantzsch thiazole synthesis.
Results: The synthesized compounds were confirmed on the basis of IR, 1H-NMR and mass analyses. The newly synthesized compounds were evaluated for their antimicrobial, anti-candidal and anti-mycobacterial activity by S. aureus and E. coli; anticandial activity of test compound was assessed against Candida albicans and Mycobacterium smegmatis for anti-mycobacterial activity.
Conclusion: The biological importance of pyridyl-thiazole derivatives is reported here in the clubbed pyridyl-thiazole derivatives as possible antimycobacterial drugs, as part of our hunt for new antitubercular medicines.
Keywords
Antimicrobial; Anti-candidal; Anti-mycobacterial; Pyridyl-thiazole
Introduction
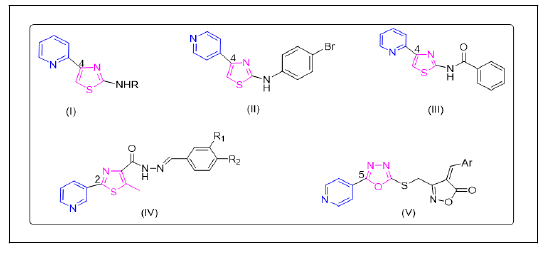
Tuberculosis (TB) is a highly contagious airborne disease caused primarily by Mycobacterium tuberculosis (Mtb), a mycobacterium species. Indeed, para-aminosalicylic acid (1946), pyrazinamide (1952), isoniazid (1952), ethambutol (1961) and rifampin (1965) were all discovered in quick succession after the discovery of TB drugs. The introduction of these antibiotics resulted in a dramatic reduction in Tuberculosis (TB) cases in developing countries. Researchers used a variety of screening techniques to find promising compound series for the development of anti-TB drugs [1]. High-throughput phenotypic screening assays have identified a large number of compound series. The Pyridyl-Thiazole (PT) and Amino-Thiazole (AT) series are promising for their activity among the compound classes identified through such phenotypic screens. Courtney C. Aldricha and co-workers discovered structure-activity correlations of 2-aminothiazoles efficient against Mycobacterium tuberculosis (I) [2]. (The paper demonstrates that analogues with a 2-pyridyl substituent are substantially more effective in GAST media, with MIC values of 0.39 M-0.78 M, which is consistent with HTS results (0.35 M for 18-19). The core aminothiazole, the 2-pyridyl substituent at C-4 and the aryl substituent at N-2 make up the lead structure. SAR revealed that the 2-pyridyl substituent at C-4 is required for efficacy, whereas N-2 aryl substituents are tolerated. Tharanikkarasu Kannan and colleagues reported on the synthesis, characterisation and in vitro and in silico analyses of 2-aminothiazole derivatives as antimycobacterial drugs (II). 4-Halophenyl at the second position of 2-aminothiazole derivatives increases activity, while 2-pyridyl at the second position of 2-aminothiazole derivatives decreases activity, according to the structure activity report. To further understand the mechanism of anti-mycobacterial action, docking experiments of these compounds with Mtb's- Ketoacyl-12 ACP synthase (KasA) protein were performed. Tanya Parish and coworkers has screened 2-aminothiazoles with excellent activity against M. tuberculosis (III). They conducted an SAR assessment of different substitutions at the C-2 and C-4 positions, as well as possible replacements for the thiazole core. 2-pyridyl moiety at the C-4 position is essential for bacterial activity, as replacement of the pyridine ring resulted in a loss of activity. The efforts around the C-2 position indicate flexibility to various modifications with amine and amide all showing activity. 4-(pyridin-2-yl)-N-(pyridin-3-yl) thiazol-2-amine was the most potent analogue prepared. Kelly=chibale and coworkers reported a series of compounds derived from the 2-amino-4-(2- pyridyl) thiazole scaffold (IV) and tested for in vitro antimycobacterial activity against the Mycobacterium tuberculosis H37 Rv strain, antiplasmodial activity against the chloroquine sensitive NF 54 Plasmodium falciparum strain and cytotoxicity on a mammalian cell line [3]. Optimal antimycobacterial activity was found with compounds with a 2- pyridyl ring at position 4 of the thiazole scaffold, a substituted phenyl ring at the 2-amino position and an amide linker between the scaffold and the substituted phenyl. The antiplasmodial activity was best with compounds that had the phenyl ring substituted with hydrophobic electron withdrawing groups. The synthesis of thiazole-based hydrazides (V) as antiinflammatory and antimicrobial agents. The in vitro antiinflammatory study of the target compounds revealed that these compounds are significant inhibitors. The in vitro antiinflammatory activity of these compounds was evaluated by denaturation of the bovine serum albumin method. Keeping in mind, the biological significance of pyridyl-thiazole derivatives and in continuation of our search for new antitubercular agents, we report here in the clubbed pyridyl-thiazole derivatives as potential antimycobacterial agents (Figure 1) [4].
Materials and Methods
Chemistry
Melting points were determined in open capillaries on a meltemp apparatus and are uncorrected. All the reactions were monitored by Thin Layer Chromatography (TLC) on precoated silica gel 60 F 254 (mesh); spots were visualised with UV light. Merck silica gel (60-120 mesh) was used for column chromatography. The UV and fluorescence were recorded on Shimadzu UV-1800 spectrophotometer. HNMR (400 MHz) and CNMR (100 MHz) spectra were recorded on a brucker avance II 400 MHz NMR spectrometer in CDCl3/DMSO-d6 solution using TMS as an internal standard. All chemical shifts were recorded in δ (ppm) using TMS as an internal standard. The mass spectra were recorded on waters, Q-TOF micromass/ESI-MS at 70 eV (Figure 2) [5].
General procedure for the synthesis of isomeric pyridine carbothioimidic acid (2,3,4): A solution of pyridine carbonitriles (5 gm/5 ml) in pyridine (15 ml) and triethyl amine (3 ml) was stirred for 15 min. H2S gas was then passed into the reaction mixture. Colour of the solution turns green. The reaction was monitored on TLC [6]. A ter stirring for about 2 hrs the solution turns to greenish yellow solid. On completion of the reaction the mixture was poured into crushed ice and stirred for the complete precipitation. The crude thioamide product was iltered and washed extensively with water. The product was recrystallized from ethanol to obtain almost pure product. Yield: 80%-90% [7].
General procedure for the synthesis of isomeric 4- phenylthiazol-2-pyridyl deriva ives (2a-h/3a-h/4a-h): To a solution of thioamide (2/3/4) (1 gm, 7.2 mmol) in ethyl alcohol (4 ml), 4-substituted phenacyl bromide (7.2 mmol) was added and the reaction was re luxed on the hot plate. A ter the completion of the reaction as monitored on TLC (3 hr), the reaction mixture was poured in cold water. The solid product obtained was iltered, washed with water, dried and recrystallized from ethanol [8].
4-phenyl-2-(pyridin-2-yl) thiazole (2a): Yield 87%, yellowish green, MP 198°C. 1H NMR (400 MHz, CDCl3): δ 7.07-δ 7.09 (m, 1H, phenyl), 7.10-7.12 (dd, J=8 Hz, J=7.6 Hz, 2H, phenyl), 7.33-7.34 (ddd, J=7.8 Hz, J=4.6 Hz, J=1.06 Hz, 1H, pyridine), 7.42 (s, 1H, thiazole), 7.76-7.79 (dt, J=1.0 and J=7.8 Hz, 1H, pyridine), 7.85-7.86 (dd, J=8 Hz, J=2.3 Hz, 2H, phenyl), 8.10-8.20 (dd, J=7.8 Hz, J=1.06 Hz, 1H, pyridine), 8.33-8.34 (dd, J=4.6 Hz, J=1.0 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 116.80, 117.29, 117.40, 121.90, 125.60, 129.02, 129.12, 139.06, 146.36, 151.43, 157.70, 164.20.
4-(4- luorophenyl)-2-(pyridin-2-yl) thiazole (2b): Yield 89%, green, MP 114°C, 1H NMR (400 MHz, CDCl3): δ 7.12-δ 7.16 (dd, J=10.64 Hz, J=8 Hz, 2H, phenyl), 7.32-7.36 (ddd, J=7.6 Hz, J=4.8 Hz, J=1.0 Hz, 1H, pyridine), 7.53 (s, 1H, thiazole), 7.80-7.85 (dt, J=1.0 Hz, J=7.6 Hz, 1H, pyridine), 7.95-7.99 (dd, J=8 Hz, J=7.44 Hz, 2H, phenyl), 8.30-8.32 (dd, J=7.92 Hz, J=1.0 Hz, 1H, pyridine), 8.62-8.63 (dd, J=4.8 Hz, J=1.0 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 114.86, 115.58, 115.79, 119.86, 124.59, 128.06, 128.14, 137.07, 149.48, 155.67 m/z (70 eV): 257.0612 (M+1).
4-(4-chlorophenyl)-2-(pyridin-2-yl) thiazole (2c): Yield 81%, light brown, MP 136°C, 1H NMR (400 MHz, CDCl3): δ 7.14-δ 7.16 (d, J=7.8 Hz, 2H, phenyl), 7.42-7.45 (ddd, J=7.6 Hz, J=4.4 Hz, J=1.02 Hz, 1H, pyridine), 7.59 (s, 1H, thiazole), 7.86-7.87 (dt, J=0.98 Hz, J=7.6 Hz, 1H, pyridine), 7.91-7.94 (d, J=7.8 Hz, 2H, phenyl), 8.33-8.34 (dd, J=7.6 Hz, J=1.02 Hz, 1H, pyridine), 8.65-8.66 (dd, J=4.4 Hz, J=0.98 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 114.86, 117.29, 118.35, 122.29, 126.77, 130.45, 132.39, 140.87, 149.38, 152.24, 158.43, 165.16.
4-(4-bromophenyl)-2-(pyridin-2-yl) thiazole (2d): Yield 52%, cream, MP 132°C, 1H NMR (400 MHz, CDCl3): δ 7.13-δ 7.17 (d, J=7.7 Hz, 2H, phenyl), 7.46-7.47 (ddd, J=7.5 Hz, J=4.2 Hz, J=1.04 Hz, 1H, pyridine), 7.60 (s, 1H, thiazole), 7.87-7.88 (dt, J=1.02 Hz, J=7.5 Hz, 1H, pyridine), 7.9-7.92 (d, J=7.7 Hz, 2H, phenyl), 8.34-8.35 (dd, J=7.5 Hz, J=1.04 Hz, 1H, pyridine), 8.64-8.65 (dd, J=4.2 and J=1.02 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 114.72, 117.08, 118.14, 121.18, 125.60, 129.39, 131.28, 138.26, 148.23, 150.00, 157.22, 164.05.
2-(pyridin-2-yl)-4-(p-tolyl) thiazole (2e): Yield 73%, yellow, MP 110°C 1H NMR (400 MHz, CDCl3): δ 6.98-δ 7.0 (d, J=8.4 Hz, 2H, phenyl), 7.30-7.31 (ddd, J=7.0 Hz, J=4.42 Hz, J=1.03 Hz, 1H, pyridine), 7.56 (s, 1H, thiazole), 7.79-7.83 (dt, J=1.01 Hz, J=7.9 Hz, 1H, pyridine), 7.92-7.94 (d, J=8.4 Hz, 2H, phenyl), 8.31-8.33 (dd, J=7.9 Hz, J=1.03 Hz, 1H, pyridine), 8.62-8.64 (dd, J=4.42 Hz, J=1.01 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 21.32 (-CH3), 113.50, 114.20, 119.80, 124.31, 125.51, 125.61, 135.82, 148.30, 150.40, 155.39, 158.20, 166.90.
4-(4-methoxyphenyl)-2-(pyridin-2-yl) thiazole (2f): Yield 90%, dark yellow, MP 108°C, 1H NMR (400 MHz, CDCl3): δ 6.96-δ 6.99 (d, J=8.8 Hz, 2H, phenyl), 7.29-7.32 (ddd, J=7.76 Hz, J=4.84 Hz, J=1.08 Hz, 1H, pyridine), 7.45 (s, 1H, thiazole), 7.78-7.82 (dt, J=1.0 Hz, J=7.76 Hz, 1H, pyridine), 7.91-7.93 (d, J=8.8 Hz, 2H, phenyl), 8.30-8.32 (dd, J=7.76 Hz, J=1.08 Hz, 1H, pyridine), 8.60-8.62 (dd, J=4.84 Hz, J=1.0 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 55.10 (-OMe), 113.60, 114.14, 119.85, 124.43, 127.51, 127.66, 136.99, 149.44, 151.55, 156.50, 159.72, 168.61 m/z (70 eV): 269.0817 (M+1).
4-(4-nitrophenyl)-2-(pyridin-2-yl) thiazole (2g): Yield 92%, yellow, MP 178°C, 1H NMR (400 MHz, CDCl3): δ 7.22-δ 7.23 (d, J=8 Hz, 2H, phenyl), 7.42-7.44 (ddd, J=7.6 Hz, J=4.42 Hz, J=1.01 Hz, 1H, pyridine), 7.92 (s, 1H, thiazole), 7.98-7.99 (dt, J=1.0 Hz, J=7.6 Hz, 1H, pyridine), 8.02-8.03 (d, J=8 Hz, 2H, phenyl), 8.42-8.44 (dd, J=7.6 Hz, J=1.01 Hz, 1H, pyridine), 8.66-8.67 (dd, J=4.42 and J=1.0 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 116.77, 117.10, 117.11, 120.82, 126.72, 130.03, 130.20, 139.12, 147.40, 152.30, 158.10, 165.20.
4-(2-(pyridin-2-yl) thiazol-4-yl) benzonitrile (2h): Yield 93%, white, MP 168°C, 1H NMR (400 MHz, CDCl3): δ 7.21-δ 7.22 (d, J=7.9 Hz, 2H, phenyl) , 7.43-7.44 (ddd, J=7.2 Hz, J=4.32 Hz, J=1.02 Hz, 1H, pyridine), 7.86 (s, 1H, thiazole), 7.78-7.79 (dt, J=1.0 Hz, J=7.2 Hz, 1H, pyridine), 8.00-8.10 (d, J=7.9 Hz, 2H, phenyl), 8.20-8.21 (dd, J=7.2 Hz, J=1.02 Hz, 1H, pyridine), 8.65-8.66 (dd, J=4.32 Hz, J=1.0 Hz, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 114.02, 114.25, 115.27, 118.70 (-CN), 124.07, 129.12, 129.24, 138.17, 146.2, 151.25, 157.06, 164.23.
4-phenyl-2-(pyridin-3-yl) thiazole (3a): Yield 98%, buff white, MP 270°C, 1H NMR (400 MHz, CDCl3): δ 7.90-δ 8.00 (m, 1H, pyridine), 8.00-8.10 (d, J=8 Hz, 2H, phenyl), 8.00-8.40 (m, 1H, pyridine), 8.00-8.90 (d, J=8 Hz, 2H, phenyl), 8.27 (s, 1H, thiazole), 8.50-8.80 (m, 1H, phenyl), 8.97 (m, 1H, pyridine), 9.51 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 115.00, 122.00, 128.00, 128.00, 131.50, 132.00, 139.00, 142.10, 144.10, 155.10, 157.10, 161.10.
4-(4- luorophenyl)-2-(pyridin-3-yl) thiazole (3b): Yield 82%, white, MP 120°C, 1H NMR (400 MHz, CDCl3): δ 7.72-7.74 (m, 1H, pyridine).
8.30-8.50 (m, J=8.04 Hz, 2H, phenyl), 8.40 (m, 1H, pyridine), 8.70 (s, 1H, thiazole), 7.90-8.00 (m, J=8.04 Hz, 2H, phenyl), 8.70-8.80 (m, 1H, pyridine), 9.10 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 117.00, 121.00, 127.10, 128.10, 131.00, 132.10, 139.70, 140.10, 144.40, 155.10, 156.40, 162.00.
4-(4-chlorophenyl)-2-(pyridin-3-yl) thiazole (3c): Yield 69%, light yellow, MP 244°C, 1H NMR (400 MHz, CDCl3): δ 7.40-δ 7.50 (d, J=8 Hz, 2H, phenyl), 8.20-8.40 (d, J=8 Hz, 2H, phenyl), 8.30 (s, 1H, thiazole), 8.03 (m, 1H, pyridine), 8.00-8.10 (m, 1H, pyridine), 8.40-8.80 (m, 1H, pyridine), 9.30 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 116.00, 120.00, 128.00, 129.80, 131.00, 133.40, 138.10, 142.00, 143.50, 154.94, 156.10, 161.00.
4-(4-bromophenyl)-2-(pyridin-3-yl) thiazole (3d): Yield 48%, light brown, MP 226°C 1H NMR (400 MHz, CDCl3): δ 7.60-δ 7.62 (d, J=8.48 Hz, 2H, phenyl), 8.00-8.05 (d, J=8.52 Hz, 2H, phenyl), 8.06-8.09 (m, 1H, pyridine), 8.27 (s, 1H, thiazole), 8.92-8.94 (m, 1H, pyridine), 8.97 (m, 1H, pyridine), 9.51 (m, 1H, pyridine).
13C NMR (100 MHz CDCl3): δ 117.20, 121.88, 127.00, 128.00, 131.51, 132.36, 139.98, 141.32, 144.50, 154.94, 156.00, 160.98 m/z (70 eV): 316.9769 (M+1) and 318.9743 (M+2).
2-(pyridin-3-yl)-4-(p-tolyl) thiazole (3e): Yield 81%, light yellow, MP 280°C, 1H NMR (400 MHz, CDCl3): δ 7.10-δ 7.15 (m, 1H, pyridine), 7.50 (s, 1H, thiazole), 7.71-7.79 (d, J=8 Hz, 2H, phenyl), 7.90-8.00 (d, J=8 Hz, 2H, phenyl), 8.00-8.09 (m, 1H, pyridine), 8.10-8.19 (m, 1H, pyridine), 9.20 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 21.32 (CH3), 117.00, 124.10, 125.10, 128.01, 130.01, 134.01, 141.02, 148.40, 152.10, 155.10, 157.00, 164.90.
4-(4-methoxyphenyl)-2-(pyridin-3-yl) thiazole (3f): Yield 97%, dark yellow, MP 204°C, 1H NMR (400 MHz, CDCl3): δ 7.40-δ 7.42 (m, 1H, pyridine), 7.43-7.46 (s, 1H, thiazole), 7.72-7.92 (d, J=8.3 Hz, 2H, phenyl), 7.90-8.00 (d, J=8.3 Hz, 2H, phenyl), 8.34-8.36 (m, 1H, pyridine), 8.71 (m, 1H, pyridine), 9.25 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 55.10 (OMe), 116.57, 122.00, 124.10, 127.00, 129.30, 132.50, 138.50, 148.00, 151.20, 153.90, 157.00, 166.00.
4-(4-nitrophenyl)-2-(pyridin-3-yl) thiazole (3g): Yield 80%, darkbrown, MP 158°C, 1H NMR (400 MHz, CDCl3): δ 7.43-δ 7.46 (m, 1H, pyridine), 7.76 (s, 1H, thiazole), 8.16-8.19 (d, J=8.9 Hz, 2H, phenyl), 8.31-8.36 (d, J=8.9 Hz, 2H, phenyl), 8.34-8.36 (m, 1H, pyridine), 8.70-8.72 (m, 1H, pyridine), 9.25-9.26 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 116.57, 123.28, 124.28, 127.07, 129.30, 133.84, 139.64, 147.78, 151.23, 154.33, 156.77 and 165.35.
4-(2-(pyridin-3-yl)thiazol-4-yl) benzonitrile (3h): Yield 75%, white, MP 260°C 1H NMR (400 MHz, CDCl3): δ 7.40 (m, 1H, pyridine), 7.73 (s, 1H, thiazole), 8.02-8.04 (d, J=8.10 Hz, 2H, phenyl), 8.10-8.20 (d, J=8.2 Hz, 2H, phenyl), 8.32-8.52 (m, 1H, pyridine), 8.50 (m, 1H, pyridine), 9.25 (m, 1H, pyridine).
13C NMR (100 MHz, CDCl3): δ 116.57, 118.10 (CN), 122.87, 123.90, 127.00, 129.00, 132.50, 139.84, 147.10, 152.00, 154.00, 156.05, 166.35.
4-phenyl-2-(pyridin-4-yl) thiazole (4a): Yield 87%, yellowish green, MP 198°C 1H MNR (400 MHz, CDCl3): δ 7.36-δ 7.40 (m, 1H, phenyl), 7.45-7.48 (d, 8.8 Hz, 2H, phenyl), 7.60 (s, 1H, thiazole), 7.89-7.90 (d, 5 Hz, 2H, pyridine), 7.97-8.00 (d, 8.8 Hz, 2H, phenyl), 8.72-8.73 (d, 5 Hz, 2H, pyridine).
13C NMR (100 MHz, CDCl3): δ 110.81, 121.32, 127.13, 128.17, 129.42, 133.40, 143.75, 149.81, 152.35, 154.92.
4-(4- luorophenyl)-2-(pyridin-4-yl) thiazole (4b): Yield 89%, green, MP 114°C 1H MNR (400 MHz, CDCl3): δ 7.11-δ 7.15 (d, J=8 Hz, 2H, phenyl), 7.52 (s, 1H, thiazole), 7.85-7.86 (d, 2H, 4 Hz, pyridine), 7.87-7.95 (dd, J=4 Hz, J=8 Hz, 2H, phenyl), 8.70-8.71 (d, 2H, 4 Hz, pyridine).
13C NMR (100 MHz, CDCl3): δ 110.85, 116.36, 121.34, 128.67, 130.61, 143.74, 149.83, 152.34, 154.94, 162.98.
4-(4-chlorophenyl)-2-(pyridin-4-yl) thiazole (4c): Yield 81%, light brown, MP 136°C, 1H MNR (400 MHz, CDCl3): δ 7.41-δ 7.45 (d, J=8.5 Hz, 2H phenyl), 7.58 (s, 1H, thiazole), 7.87-7.88 (d, J=6.12 Hz, 2H, pyridine), 7.90-7.94 (d, J=8.5 Hz, 2H, phenyl), 8.72-8.74 (d, J=6.12 Hz, 2H pyridine).
13C NMR (100 MHz, CDCl3): δ 110.85, 121.30, 128.95, 129.37, 131.15, 134.34, 143.76, 149.86, 152.37, 154.94.
4-(4-bromophenyl)-2-(pyridin-4-yl) thiazole (4d): Yield 52%, cream, MP 132°C, 1H MNR (400 MHz, CDCl3): δ 7.57-δ 7.58 (d, J=9 Hz, 2H, phenyl), 7.59 (s, 1H, thiazole), 7.85-7.86 (d, J=6.16 Hz, 2H, pyridine), 7.87-7.89 (d, J=9 Hz, 2H, phenyl), 8.72-8.74 (d, J=6.16 Hz, 2H, pyridine).
13C NMR (100 MHz, CDCl3): δ 110.81, 121.20, 123.15, 128.34, 132.08, 132.15, 143.74, 149.87, 152.34, 154.56.
2-(pyridin-4-yl)4-(p-tolyl)-thiazole (4e): Yield 73%, yellow, MP 110°C, 1H MNR (400 MHz, CDCl3): δ 7.25-δ 7.27 (d, J=5.8 Hz, 2H, pyridine), 7.52 (s, 1H, thiazole), 7.86-7.87 (d, J=8.16 Hz, 2H, phenyl), 7.87-7.88 (d, J=8.16 Hz, 2H, phenyl), 8.70-8.72 (d, J=6.16 Hz, 2H, pyridine).
13C NMR (CDCl3) δ 110.86, 121.00, 123.02, 128.22, 131.08, 132.05, 143.44, 149.57, 152.44, 154.38, 21.33 (-CH3). m/z (70 eV): 253.0790 (M+1).
4-(4-methoxyphenyl)-2-(pyridin-4-yl) thiazole (4f): Yield 90%, dark yellow, MP 108°C, 1H MNR (400 MHz, CDCl3): δ 6.97-δ 6.99 (d, J=8.84 Hz, 2H phenyl), 7.45 (s, 1H, thiazole), 7.87-7.88 (d, J=6.12 Hz, 2H, pyridine), 7.90-.93 (d, J=8.84 Hz, 2H, phenyl), 8.70-8.72 (d, J=6.12 Hz, 2H, pyridine).
13C NMR (100 MHz, CDCl3): δ 55.37 (OMe), 112.61, 114.21, 120.34, 126.93, 127.82, 140.47, 150.64, 157.00, 159.97, 164.67. m/z (70 eV): 269.0733 (M+1).
4-(4-nitrophenyl)-2-(pyridin-4-yl) thiazole (4g): Yield 92%, MP 178°C, 1H MNR (400 MHz, CDCl3): δ 7.82 (s, 1H, thiazole), 7.907.91 (d, J=6.16 Hz, 2H, pyridine), 8.16-8.19 (d, J=9.2 Hz, 2H, phenyl), 8.32-8.35 (d, J=9.2 Hz, 2H, phenyl), 8.76-8.77 (d, J=6.16 Hz, 2H, pyridine).
13C NMR (100 MHz, CDCl3): δ 110.47, 121.33, 124.11, 126.23, 139.03, 143.11, 147.57, 149.48, 152.63, 154.45.
4-(2-(pyridin-4-yl)thiazole-4-yl)-benzonitrile (4h): Yield 93%, white, MP 168°C, 1H MNR (400 MHz, CDCl3): δ 7.74-δ 7.77 (d, J=8.4 Hz, 2H, phenyl), 7.76 (s, 1H, thiazole), 7.88-7.90 (d, J=6.12 Hz, 2H, pyridine), 8.10-8.12 (d, J=8.4 Hz, 2H, phenyl), 8.75-8.76 (d, J=6.12 Hz, 2H, pyridine).
13C NMR (100 MHz, CDCl3): δ 110.11, 112.66, 118.66 (CN), 121.23, 126.05, 132.41, 137.63, 143.47, 149.58, 152.43, 154.95.
In vitro pharmacology
Culture collection and maintenance: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853 were obtained from BAC-TEST laboratory, Nasik. Clinical isolates of Candida albicans, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae were obtained from the BACTEST laboratory, Nasik. These cultures were grown on Nutrient Agar (NA) and yeast was grown Sabourauds Dextrose Agar (SDA) and incubated at 37°C for 24 hours. Mycobacterium smegmatis NCIM 5138 was procured from NCIM, National chemical laboratory, Pune and maintained on Mycobacterium phlei medium and incubated at 37°C for 72 hours [9].
Antibiotics and test compound used: Standard antibiotic disc impregnated with gentamicin (10 mg), imipinem (10 mg), penicillin (10 mg) were obtained from Hi-media, Mumbai for testing the antibiogram against standard cultures. Itraconazole, combutol-microzide and 6 pyridyl thiazole variants viz. 2b, 3b, 4b and 2f, 3f, 4f.
Solubility testing: Solvents: Water, ethyl acetate (SDFCL, Mumbai), hexane (Hi-media, Mumbai), benzene (Research lab, Mumbai) and dimethyl sulfoxide (Hi-media, Mumbai) were screened for testing the solubility of 4-pyridyl compound variants (NO2, CN, Cl, F, Br, OMe, Me). The solubility of 6 pyridyl thiazole variants test were assessed in variant solvents: Ethyl acetate (Hi-media, Mumbai), methanol (Rankem, Thane), ethanol (Merck Mumbai), diethyl ether (Hi-media, Mumbai), DMSO, petrolium ether (Hi-media, Mumbai), cyclohexane (Ranbaxy), chloroform (Hi-media, Mumbai), acetone (Hi-media, Mumbai), dichloromethane (Hi-media, Mumbai), toluene (Merck, Mumbai) and Dimethylformamide (DMF) (Hi-media, Mumbai). A pinch of test compound was dissolved in 1 ml of solvent [10].
Preparation of 0.5 McFarland standard: A volume of 0.5 ml of 0.048 mol/L BaCl2 was added to 99.5 ml of 0.18 mol/L H2SO4 (1% v/v) with constant stirring to maintain suspensions. Absorbance at 625 nm should be 0.008 to 0.10 for 0.5 McFarland standard.
Anti-bacterial screening: The newly synthesized and pure 4- pyridyl compound variants were evaluated for their antibacterial activity against Esherichia coli ATCC 215922 and Staphylococcus aureus ATCC 25923. Two fold serial dilutions (1024 μg/ml, 512 μg/ml, 256 μg/ml, 128 μg/ml, 64 μg/ml, 32 μg/ml, 16 μg/ml, 8 μg/ml) of 4-pyridyl variants (NO, CN, Cl, OMe, Me, Br, F) were made in sterile Muller-Hinton Broth (MHB) inoculated with 15 μl of log phase culture of Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 culture adjusted to 0.5 McFarland standard that is 1.5 × 108 CFU/ml incubated at 37°C for 24 hours. Next day, the tubes were examined visually for growth (turbidity) and no growth (no turbidity) [11]. The highest dilution inhibiting the growth was recorded as the Minimum Inhibitory Concentration (MIC). A loopful from the highest dilution was streaked on sterile Muller-Hinton Agar (MHA) plates which did not show growth a ter incubation was recorded as Minimum Bactericidal Concentration (MBC). The concentration which did not inhibit the growth was recorded as Sub-Inhibitory Concentration (SIC). Antibacterial activity of test compound was assessed against two standard cultures (one gram positive Staphylococcus aureus ATCC 25923 and one gram negative Escherichia coli ATCC 25922). Tubes containing sterile 20 ml of MHA medium were seeded with 0.1 ml log phase culture adjusted to 0.5 McFarland standard when the temperature of the medium reached 40°C-50°C and were plate out in sterile empty petri plates. The media was allowed to solidify and wells of 6 mm diameter were punched with the help of sterile cork borer [12]. Each well was illed with (50 μl) test compounds. The solutions of the test compound with concentration of 10 mg/ml were prepared in ethyl acetate and DMSO respectively. The disks of penicillin (10 mg/ml) and gentamicin (10 mg/ml) were also placed on agar medium for comparison. The plates were kept in the refrigerator for 15 minutes for prediffusion and then incubated at 37°C for 24 hours. Solvent without test compound served as a control. The sensitivities of the microorganisms were determined by measuring the zone of inhibition (including the diameter of the well) on the agar surface around the wells.
Inoculums development and growth curve of Candida albicans: Single colony of Candida albicans culture grown on SDA plate for 24 hours at 37°C was inoculated in 25 ml SD broth and incubated at 37°C for 32 hours. At an interval of 1 hour, the aliquots were withdrawn and Optical Density (OD) was determined at 530 nm and the total viable cell count was estimated using saline by serial dilution method. From each dilution, 0.1 ml was spread onto SDA plates and incubated at 37°C for 24 hours. The total number of colonies was enumerated in each plate [13].
Pharmacological evaluation
Anti-candidal activity: The newly synthesized and pure 4- pyridyl compound variants were evaluated for their anticandidal activity against Candida albicans. Two fold serial dilutions (1024 μg/ml, 512 μg/ml, 256 μg/ml, 128 μg/ml, 64 μg/ml, 32 μg/ml, 16 μg/ml, 8 μg/ml) of 4-pyridyl variants (NO, CN, Cl, OMe, Me, Br, F) were made in sterile Sabouraud Dextrose Broth (SDB) inoculated with 15 μl of log phase culture adjusted to 0.5 McFarland standard that is 1.5 × 106 CFU/ml incubated at 37°C for 24 hours [14]. Next day, the tubes were examined visually for growth (turbidity) and no growth (no turbidity). The highest dilution inhibiting the growth was recorded as the Minimum Inhibitory Concentration (MIC). A loopful from the highest dilution was streaked on sterile Sabourauds Dextrose Agar (SDA) plates which did not show growth after incubation was recorded as Minimum Fungicidal Concentration (MFC). The concentration which did not inhibit the growth was recorded as Sub-Inhibitory Concentration (SIC). Anticandial activity of test compound was assessed against Candida albicans. Tubes containing sterile 20 ml of sterile SDA medium were seeded with 0.1 ml log phase culture adjusted to 0.5 McFarland standards when the temperature of the medium reached 40°C-50°C and were platted out in sterile empty petri plates. The media was allowed to solidify and wells of 6 mm diameter were punched with the help of sterile cork borer. Each well was filled with (50 μl) test compounds. The solutions of the test compound with concentration of 10 mg/ml were prepared in ethyl acetate and DMSO respectively [15]. The disks of itraconazole were also placed on agar medium for comparison. The plates were kept in the refrigerator for 15 minutes for prediffusion and then incubated at 37°C for 24 hours. Solvent without test compound served as a control. The sensitivities of the microorganisms were determined by measuring the zone of inhibition (including the diameter of the well) on the agar surface around the wells.
The newly synthesized pyridyl thiazole compound variants were evaluated for their anti- candidal activity against Candida albicans. The Minimum Inhibitory Concentration (MIC), Minimum Fungicidal Concentration (MFC) and Sub-Inhibitory Concentration (SIC) of the pyridyl thiazole compound were determined. Two fold serial dilutions (4096 μg/ml, 2048 μg/ml, 1024 μg/ml, 512 μg/ml, 256 μg/ml, 128 μg/ml, 64 μg/ml, 32 μg/ml, 16 μg/ml, 8 μg/ml, 4 μg/ml, 2 μg/ml, 1 μg/ml and 0.5 μg/ml) of pyridyl thiazole variants were made in sterile Sabouraud Dextrose (SD) broth were inoculated with 100 μl of log phase culture adjusted to approximately 1 × 106 CFU/ml by optical density method and incubated at 37°C for 24 hours [16]. Next day, the tubes were examined visually for turbidity indicating growth and no turbidity indicating no growth. The highest dilution inhibiting the growth was recorded as the Minimum Inhibitory Concentration (MIC). A loopful from the higher dilutions was streaked on sterile Sabouraud Dextrose Agar (SDA) plates and the one which did not exhibit growth after incubation was recorded as Minimum Fungicidal Concentration (MFC). The highest concentration which did not inhibit the growth prior to MIC was recorded as Sub-Inhibitory Concentration (SIC). For determining the anti-candidal activity of the test compound against Candida albicans, Agarwell diffusion method was performed. Tubes containing 20 ml of sterile SDA medium (at 40°C) were seeded with 0.1 ml log phase culture and poured in sterile empty petri plates. The media was allowed to solidify and wells of 7 mm diameter were punched with the help of sterile cork borer. Each well was illed with 50 μl test compounds as solutions with their MFC concentration in acetone, DMF and ethanol respectively. The plates were kept in the refrigerator for 15 minutes for pre-diffusion and then incubated at 37°C for 24 hours. Solvent without test compound served as a control. The sensitivities of the microorganisms were determined by measuring the zone of inhibition on the agar surface around the wells [17].
Inoculum development and growth curve of Mycobacterium smegmatis: Single colony of Mycobacterium smegmatis culture grown on Mycobacterium Phlei Agar (MPA) for 72 hours at 37°C was inoculated in 25 ml MP broth and incubated at 37°C for 81 hours. At an interval of 3 hour, the aliquots were withdrawn for the total viable cell count by spreading 0.1 ml culture onto MP agar plates in triplicate and incubated at 37°C for 72 hours. The total number of colonies was enumerated in each plate.
Anti-mycobacterial activity: The variations of the freshly synthesised and pure 4-pyridyl molecule were assessed for their antimycobacterial activity against Mycobacterium smegmatis NCIM 5138. Two-fold serial dilutions of 4-pyridylvariants (NO, CN, Cl, OMe, Me, Br, F) were produced in sterile Mycobacterium phlei media and infected with 15 l of log phase culture containing 5 103 CFU/ml and incubated at 37°C for 24 hours. The tubes were visually inspected the following day for growth (turbidity) and absence of growth (no turbidity). The Minimum Inhibitory Concentration was determined to be the highest dilution that inhibited growth (MIC). A loopful from the highest dilution was streaked on sterile Mycobacterium Phlei Agar (MPA) plates, and the amount that did not show growth a ter incubation was recorded as the Minimum Bactericidal Concentration (MBC). Sub-Inhibitory Concentration was assigned to the concentration that did not prevent growth (SIC). Test substance's antimycobacterial activity was evaluated against Mycobacterium smegmatis NCIM 5138. On sterile MPA, 0.1 cc of culture was smeared. A sterile cork borer was used to drill 6 mm diameter wells. Test compounds were poured into each well. In DMSO and ethyl acetate, respectively, the test chemical solutions with a concentration of 10 mg/ml were made. To provide a baseline for comparison, the combutolmicrozide discs were also put on an agar medium [18]. 48 hours of incubation at 37°C followed by a prediffusion period of 15 minutes in the refrigerator were used for the plates. Control solvent was one without the test substance. The sensitivity of the bacteria was determined by measuring the zone of inhibition, which encompasses the diameter of the well, on the agar surface around the wells. Mycobacterium smegmatis NCIM 5138 was used to test the anti-mycobacterial activity of the newly synthesised pyridyl thiazole variants. The Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and Sub-Inhibitory Concentration (SIC) of pyridyl thiazole were determined. To sterile Mycobacterium phlei media, 100 l of a log phase culture with 1-10-3-5-10-3 CFU/ml was added. It was then incubated at 37°C for 72 hours. The following quantities of two fold serially diluted pyridyl thiazole variants were made: 2048, 1024, 512, 4096, 2048 and after the tubes had been incubated, they were visually examined for turbidity, which indicates growth and absence of turbidity, which indicates no growth. The greatest dilution that stopped growth was recorded as the Minimum Inhibitory Concentration (MIC). The highest dilution was chosen from a loopful of the highest dilutions and streaked onto sterile Mycobacterium Phlei Agar (MPA) plates. This dilution was then incubated and the absence of growth was recorded as the highest dilution. The Sub-Inhibitory Concentration was found to be the maximum concentration that did not hinder growth before the MIC (SIC). Utilizing the agar well diffusion method, the test substance's potency against Mycobacterium smegmatis NCIM 5138 was assessed [19]. The log phase culture was seeded into tubes holding 20 ml of sterile MPA medium in sterile empty petri dishes using 0.1 ml of the culture. 40°C was used for this operation. In order to drill the 7 mm-diameter wells, a sterile cork borer was used after the media had had time to harden. 50 l of test chemicals were placed into each well in solutions with the appropriate MBC concentrations in acetone, DMF and ethanol. The plates underwent pre-diffusion for 15 minutes in the refrigerator before being incubated at 37°C for 72 hours. An ineffective solution was controlled. The sensitivity of the bacteria was determined by measuring the zone of inhibition on the agar surface surrounding the wells.
Determination of MIC and FIC Index (FICI): The overall impact of the pyridyl thiazole variations (2 PT-OMe, 4 PT-OMe, 2 PT-F and 4 PT-F) was evaluated using the two-fold serial dilution method. 4 combinations were created using these 4 variants. The combinations are 2 PT-F with 2 PT-OMe, 2 PT-F with 4 PTOMe, 4 PT-F with 2 PT-OMe and 4 PT-F with 4 PT-OMe. For their effectiveness against Candida albicans, these 4 combinations were tested. In sterile Sabourauds Dextrose Broth (SDB) injected with 100 l of log phase culture 1 × 106 CFU/ml incubated at 37°C for 24 hours for Candida albican two-fold serial dilutions (4096 g/ml, 2048 g/ml, 512 g/ml, 128 g/ml, 64 g/ml, 32 g/ml. The formula below was used to compute FICI values for each combination:

Interactions were determined by assigning values calculated in the following way:
Synergism-FIC index<0.5
Indifference-FIC index 0.5-4
Antagonism-FIC index>4
Results and Discussion
Anti-bacterial activity
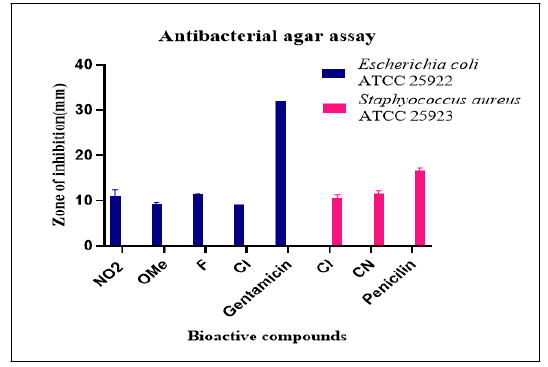
Agar well diffusion: Using the agar well diffusion method, it was determined what these variants' potential activities, as shown in Figure 1, might be. Test versions' antibacterial abilities were evaluated by measuring the zone widths and comparing the outcomes with those of well-known drugs (standard) as displayed [20]. Cl substituted variants (2c, 3c and 4c) of the tested variations shown activity against both S. aureus and E. coli, though it was found that the test was more active. F-variant (2b, 3b and 4b) revealed stronger inhibitory activity against E. coli than other variations that demonstrated intermediate inhibitory action, nevertheless. The test versions could reduce bacteria, although their efficacy was less than anticipated (Table 1).
| 4-Pyridyl variants | Name of the bacteria | |||
|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Staphylococcus aureus ATCC 23923 | |||
| Zone of inhibition (mm) | S/I/R | Zone of inhibition (mm) | S/I/R | |
| 4a (H) | 0 | R | 0 | R |
| 4b (F) | 11.25 ± 0.35 | R | 0 | R |
| 4c (Cl) | 9 | R | 10.5 ± 0.7 | R |
| 4d (Br) | 0 | R | 0 | R |
| 4e (Me) | 0 | R | 0 | R |
| 4f (OMe) | 9.25 ± 0.35 | R | 0 | R |
| 4g (NO2) | 11 ± 1.41 | R | 0 | R |
| 4h (CN) | 0 | R | 11.5 ± 0.7 | R |
| Standard | 31.3 ± 1.15 | S | 16.5 ± 0.7 | R |
Table 1: Anti-bacterial activity by agar well diffusion.
Broth dilution: Antibacterial screening data analysis revealed that variant 4b (F) had the highest level of inhibitory action,follo -wed by variations (4g) NO2, (4f) OMe, (4h) CN and (4c) Cl, while variations (4d) Br and (4e) Me had the lowest inhibitory activity [21]. It was discovered that the MBC and MIC were the same for the majority of the compounds. MIC values for variant (4b) F ranged from 512 to 1024 g/ml, however they were lower than the standard due to the variation's higher level of inhibition (Table 2 and Figure 3).
| 4-Pyridyl variants | Escherichia coli ATCC 25922 | Staphylococcus aureus ATCC 25923 | ||||
|---|---|---|---|---|---|---|
| MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | |
| 4a (H) | R | R | R | R | R | R |
| 4b (F) | 1024 | 512 | 256 | >1024 | 1024 | 512 |
| 4c (Cl) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4d (Br) | R | R | R | R | R | R |
| 4e (Me) | R | R | R | R | R | R |
| 4f (OMe) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4g (NO2) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4h (CN) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| Standard antibiotic | 8 | <4 | 2 | 0.5 | >0.25 | 0.125 |
Table 2: Anti-bacterial activity using broth dilution.
Anti-candidal activity
Agar well diffusion: Table 3 demonstrates all the variations showed some anticandidal activity, but methoxy and fluoride versions showed the most promise. The variation 4f (OMe) was also discovered to be almost as potent as the standard treatment itraconazole. For the other variations, Candida albicans was found to be resistant. Similar effects to itraconazole are also being shown by derivative 3b (F). The third photo plate test substances' anti Candida albicans activity, a conventional drug's anti-candida properties, and the anti-candida properties of pyridyl thiazole variations in vitro (Table 3).
| Derivative | Candida albicans/Zone of inhibition (mm) |
|---|---|
| p-Fluoride substituent | |
| 2b | 8.5 ± 0.5 |
| 3b | 15.34 ± 1.53 |
| 4b | 8.67 ± 0.58 |
| p-Methoxy substituent | |
| 2f | 10.34 ± 0.58 |
| 3f | 9.17 ± 0.29 |
| 4f | 8.5 ± 0.5 |
| Standard | 11.5 ± 0.5 |
| Acetone | 7 |
| DMF | 11.5 ± 0.71 |
| Ethanol | 8 ± 0.71 |
Table 3: Anti-candidal activity using agar well diffusion.
Broth dilution: MBCs for the majority of the compounds were discovered to match MICs exactly. Variant F was inferior to the benchmark even though it showed the greatest inhibition at MIC values between 512 g/ml and 1024 g/ml. Additionally, every version of OMe and F showed support for trump (Table 4). The most effective variant against the test organism was found to be 3b (F). It was found that the variants 2f (OMe) and 3b (F) have approximately the same potency as the reference drug itraconazole (Table 4).
| Derivative | Candida albicans | ||
|---|---|---|---|
| MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | |
| p-Fluoride substituent | |||
| 2b | 1024 | 512 | 256 |
| 3b | 512 | 256 | 128 |
| 4b | 1024 | 512 | 256 |
| p-Methoxy substituent | |||
| 2f | 4096 | 2048 | 1024 |
| 3f | 2048 | 1024 | 512 |
| 4f | 2048 | 1024 | 512 |
| Standard antibiotic | 2 | >1 | 0.5 |
| Itraconazole | 2 | >1 | 0.5 |
Table 4: Anti-candidal activity using broth dilution.
Inoculum development and growth curve of Mycobacterium smegmatis: The growth curve must be established by inoculating viable cells into broth medium and allowing them to proliferate under optimum circumstances. According to the findings, the lag time for Candida albicans was about 10 hours. A 16 hour-30 hour log phase was experienced by Candida albicans. The OD was 1 to 1.5, and the cell count varied between 1 106 and 5 106 between 16 and 20 hours. The incubation time and cell count as a result.
Anti-mycobacterial assay
Broth dilution: Amongst the variants 3f (OMe). It was found to be most active against test organism. Fluoride containing derivatives has similar MIC concentration. None of the drug has MIC equal or less than reference drug (Table 5).
| Derivative | Mycobacterium smegmatis NCIM 5138 | ||
|---|---|---|---|
| MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | |
| p-Fluoride substituent | |||
| 2b | 1024 | 512 | 256 |
| 3b | 1024 | 512 | 256 |
| 4b | 1024 | 512 | 256 |
| p-Methoxy substituent | |||
| 2f | 2048 | 1024 | 512 |
| 3f | 256 | 128 | 4 |
| 4f | 2048 | 1024 | 512 |
| Standard antibiotic | 1024 | 512 | 256 |
| Combutol | 8 | 4 | 2 |
Table 5: Anti-mycobacterial activity using broth dilution method.
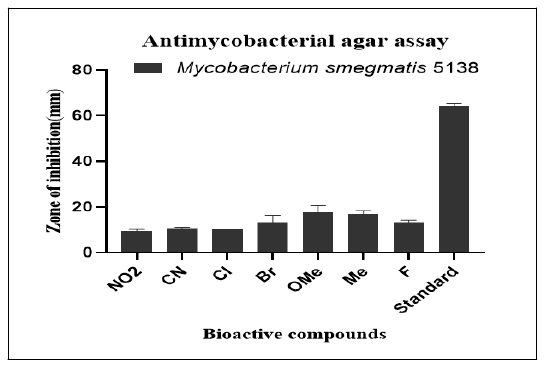
Agar well diffusion: The results of the antimycobacterial screening investigation showed that Me and Br were the two variations that showed the highest levels of inhibition, followed by OMe. Even while they showed growth inhibition of the test organism, it was far less than the required level. Agar well diffusion's anti-mycobacterial effectiveness (Tables 6 and 7).
| 4-Pyridyl variants | Mycobacterium smegmatis NCIM 5138 | |
|---|---|---|
| Zone of inhibition (mm) | S/I/R | |
| 4b (F) | 13.25 ± 0.75 | R |
| 4c (Cl) | 10 ± 0 | R |
| 4d (Br) | 13.25 ± 2.25 | R |
| 4e (Me) | 17 ± 1 | R |
| 4f (OMe) | 17.6 ± 2.1 | R |
| 4g (NO2) | 9.5 ± 0.5 | R |
| 4h (CN) | 10.5 ± 0.5 | R |
| Standard | 64.25 ± 0.75 | S |
Table 6: Anti-mycobacterial activity using agar well diffusion method.
| 4-Pyridyl variants | Mycobacterium smegmatis NCIM 5138 | |||||
|---|---|---|---|---|---|---|
| MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | MBC (µg/ml) | MIC (µg/ml) | SIC (µg/ml) | |
| 4a (H) | - | - | - | - | - | - |
| 4b (F) | 1024 | 512 | 256 | >1024 | 1024 | 512 |
| 4c (Cl) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4d (Br) | R | R | R | R | R | R |
| 4e (Me) | R | R | R | R | R | R |
| 4f (OMe) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4g (NO2) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| 4h (CN) | >1024 | 1024 | 512 | >1024 | 1024 | 512 |
| Standard antibiotic | 8 | <4 | 2 | 0.5 | >0.25 | 0.125 |
Table 7: Anti-mycobacterial activity using broth dilution method.
Determination of MIC and FIC Index (FICI): Only one combination, 4 PT-F (4b)+4 PT-OMe (4f) for Candida albicans, exhibits synergistic interaction, while the other three combinations exhibit indifferent interaction (Table 8 and Figure 4).
| Combinations (Drug 1+Drug 2) | Candida albicans | |||
|---|---|---|---|---|
| FIC drug 1 | FIC drug 2 | FICI | Interaction | |
| 4b+2f | 0.5 | 0.125 | 0.625 | IND |
| 4b+4 f | 0.25 | 0.125 | 0.375 | SYN |
| 2b+2f | 0.5 | 0.125 | 0.625 | IND |
| 2b+4f | 1 | 0.5 | 1.5 | IND |
Table 8: Fractional Inhibitory Concentration Index (FICI) for combinations of pyridyl thiazole variants.
Conclusion
A facile approach was used to successfully synthesise three series of isomeric pyridyl-thiazole derivatives. Using the agar well diffusion and broth dilution methods, anti-bacterial, anticandidal and anti-mycobacterial activity was evaluated. Among the three isomeric series, moderate activity were identified for the 4-pyridyl versions with (4b) F, (4c) Cl, (4f) OMe, (4g) NO2 and (4h) CN. Next, research on pyridyl-variants with fluorine and methoxy substituents was concentrated for their anticandidal and antimycobacterial properties. The pyridine's 4-position is often more active than its 2- and 3-positions. For more research to confirm the trend mentioned in this paper, compounds 4b and 4f can act as lead molecules.
Acknowledgement
The authors are thankful to management and principal of, HPT Arts and RYK Science College, Nashik, MS, India for providing all necessary facility to carry out the research.
Conflict of Interest
Author declares no conflict of interest.
References
- Pethe K, Sequeira PC, Agarwalla S, Rhee K, Kuhen, K, et al. (2010) A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat Comm 1: 57.
[Crossref] [Google Scholar] [PubMed]
- Ballell L, Bates RH, Young RJ, Alvarez Gomez D, Alvare Ruiz E, et al. (2013) Fueling open source drug discovery: 177 small molecule leads against tuberculosis. Chem Med Chem 8: 313-321.
[Crossref] [Google Scholar] [PubMed]
- Farouk EM, Mohamed AB, Fawzi FM (2021) An overview on synthetic 2-aminothiazole-based compounds associated with four biological activities. Molecules 26: 1449.
[Crossref] [Google Scholar] [PubMed]
- Ananthan S, Faaleolea ER, Goldman RC, Hobrath JV, Kwong CD, et al. (2009) High-throughput screening for inhibitors of Mycobacterium tuberculosis H37 Rv. Tuberculosis 89: 334-353.
[Crossref] [Google Scholar] [PubMed]
- Meissner A, Boshoff HI, Vasan M, Duckworth BP, Barry III CE, et al. (2013) Structure activity relationships of 2-aminothiazoles effective against Mycobacterium tuberculosis. Bioorg Med Chem 21: 6385-6639.
[Crossref] [Google Scholar] [PubMed]
- Makam P, Kannan T (2014) 2-Aminothiazole derivatives as antimycobacterial agents: Synthesis, characterization, in vitro and in silico studies. Euro J Med Chem 87: 643-656.
[Crossref] [Google Scholar] [PubMed]
- Kesicki EA, Bailey MA, Ovechkina Y, Early V, Alling T, et al. (2016) Synthesis and evaluation of the 2-aminothiazoles as anti-tubercular agents. PLoS One 11: e0155209.
[Crossref] [Google Scholar] [PubMed]
- Mjambili F, Njoroge M, Naran K, De Kock C, Smith PJ, et al. (2014) Synthesis and biological evaluation of 2-aminothiazole derivatives as antimycobacterial and antiplasmodial agents. Bioorgan Med Chem Letter 24: 560-564.
- Jordan P, Costa A, Specker E, Popp O, Volkamer A, et al. (2023) Small molecule inhibiting microglial nitric oxide release could become a potential treatment for neuroinflammation. Plos One 18: e0278325.
- Suryawanshi M, Patil A, Bholay A, Bobade V (2018) Metal free synthesis, docking studies and antimicrobial screening of novel isomeric pyridine substituted thiazole derivatives. Ind J Chem.
- Suryawanshi M, Gujar V, Ottoor D, Bobade V (2019) Synthesis, characterization and photophysical properties of novel thiazole substituted pyridine derivatives.
- Jadav SS, Badavath VN, Ganesan R, Ganta NM, Besson D, et al. (2020) Biological evaluation of 2-aminothiazole hybrid as antimalarial and antitrypanosomal agents: Design and synthesis. Anti-Infect Agen 18: 101-108.
- Mjambili F, Njoroge M, Naran K, De Kock C, Smith PJ, et al. (2014) Synthesis and biological evaluation of 2-aminothiazole derivatives as antimycobacterial and antiplasmodial agents. Bioorg Med Chem Letter 24: 560-564.
[Crossref] [Google Scholar] [PubMed]
- Kamat V, Santosh R, Poojary B, Nayak SP, Kumar BK, et al. (2020) Pyridine-and thiazole-based hydrazides with promising anti-inflammatory and antimicrobial activities along with their in silico studies. ACS Omega 5: 25228-25239.
[Crossref] [Google Scholar] [PubMed]
- Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, et al. (2011) Clinical effect of point mutations in myelodysplastic syndromes. New Eng J Med 364: 2496-2506.
[Crossref] [Google Scholar] [PubMed]
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP (1988). Hospital-acquired candidemia: The attributable mortality and excess length of stay. Arch Intern Med 148: 2642-2645.
[Crossref] [Google Scholar] [PubMed]
- Marques GH, Kunzler A, Bareno DV, Drawanz B, Mastelloto H, et al. (2014) Antifungal activity of 3-(heteroaryl-2-ylmethyl) thiazolidinone derivatives. Med Chem 10: 355-360.
- Carradori SC, Secci D, Bolasco A, Rivanera D, Mari E, et al. (2013) Synthesis and cytotoxicity of novel (thiazol-2-yl) hydrazine derivatives as promising anti-candida agents. Euro J Med Chem 65: 102-111.
[Crossref] [Google Scholar] [PubMed]
- Guinet R, Nerson D, de Closets F, Dupouy-Camet J, Kures L, et al. (1988) Collaborative evaluation in seven laboratories of a standardized micromethod for yeast susceptibility testing. J Clin Microbiol 26: 2307-2312.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Rendon E, Cerna-Cortes JF, Ramirez-Medina MA, Helguera-Repetto AC, Rivera-Gutierrez S, et al. (2012) Mycobacterium mucogenicum and other non-tuberculous mycobacteria in potable water of a trauma hospital: A potential source for human infection. J Hosp Infec 80: 74-76.
[Crossref] [Google Scholar] [PubMed]
- Fratini F, Mancini S, Turchi B, Friscia E, Pistelli L, et al. (2017) A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium JR et G. Forst essential oils against Staphylococcus aureus strains. Microbiol Res 195: 11-17.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences