DTAB Catalysed Azadirachta indica Oil/ Water Bio-Emulsion for Enhancing the Antioxidant Efficacy of Ceftriaxone
Ameta RK ,Sangani CB, Pansuriya B, Mali M, Kataria P, Patel M and Kawad M
DOI10.21767/2469-6692.10022
Ameta RK*, Sangani CB, Pansuriya B, Mali M, Kataria P, Patel M and Kawad M
Department of Chemistry, KSV University, Gandhinagar, Gujarat, India
- *Corresponding Author:
- Ameta RK
Senior Research Associate
Department of Chemistry
KSV University
Gandhinagar, Gujarat, India
Tel: 079-23244690
E-mail: ametarakesh40@gmail.com
Received Date: September 13, 2017; Accepted Date: September 19, 2017; Published Date: September 29, 2017
Citation: Ameta RK, Sangani CB, Pansuriya B, Mali M, Kataria P, et al. (2017) DTAB Catalysed Azadirachta indica Oil/ Water Bio-Emulsion for Enhancing the Antioxidant Efficacy of Ceftriaxone. J In Silico In Vitro Pharmacol. 3:22. doi: 10.21767/2469-6692.10022
Abstract
For examining the improvement in antioxidant aptitude of Ceftriaxone, an antibiotic drug, through encapsulation, the bio-emulsion using Neem oil (Azadirachta indica) with aqueous Dodecyl Trimethyl Ammonium Bromide (DTAB) have been framed. The prepared emulsions have been characterized with physicochemical properties (PCPs) where alterations in PCPs of pure and drug encapsulated emulsions were attributed for distinguishing them. The formulations have been screened for radical scavenging activity (RSA) with free radical 2, 2-diphenyl-1-picrylhydrazyl through spectrophotometric and physicochemical methods. With DPPH (2,2-diphenyl-1- picrylhydrazyl) assay, the antioxidant potential of pure drug and with emulsion have been carried out where the formulation showed increment in such activity of ceftriaxone. First time the scavenging activity is examined with PCPs along with DPPH assay where reduction in values of surface tension, viscosity and potential are attributed for the radical scavenging activity, similar to DPPH assay. The reduced values of such properties of ceftriaxone encapsulated emulsions have quantitatively confirmed the improvement of antioxidant activity of ceftriaxone.
Keywords
Ceftriaxone; Surfactants; Emulsions; Neem oil; DPPH
Introduction
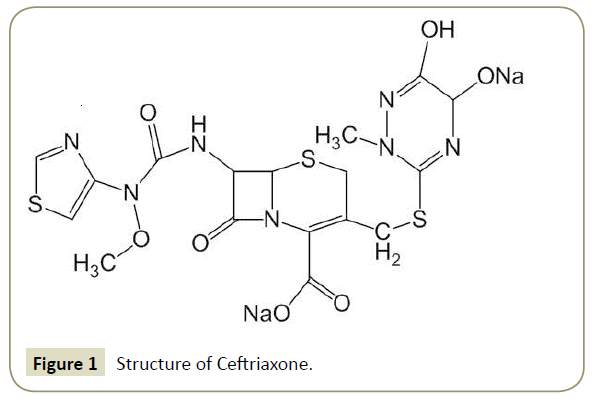
Several studies report neem oil (Azadirachta indica) as bioactive compounds, and that has been found to be insecticidal resistant [1-4]. Not only neem oil but also the neem products have also been investigated as antimalarial disease [5-7]. The formulations comprising various neem products have been found effective including in aqueous or organic phases [7]. Mentioned activities fascinated us to choose the neem oil for the emulsion formulation in the present study to evaluate the antioxidant ability of Ceftriaxone, an antibiotic drug via encapsulation. The sodium salt of Ceftriaxone (Figure 1) is a semi- synthetic antibiotic, and derivatives of 7-aminocephalosporic acid [8-12]. It is an extensive acting antibiotic for parenteral use, and shows in vitro antimicrobial activity against a long variety of microorganisms and its stability towards Gram-positive and Gram-negative bacteria, is higher [13-18]. Thus, the trend is that the many industries and academician are looking for the same and its derivatives [19- 22]. Structurally, the presence of nitrogen containing six and five membered rings and carbonyl moiety, impart Ceftriaxone an electrophilic character, thereby making this a proficient free radical scavenger. Such activities are enhanced by applying some other sources, like, the emulsions, have proven effective and reliable haulers due to their flexible compositions in terms of hydrophobic and hydrophilic behavior [23-27]. Such formulations grip immense possibilities for improving beneficial efficacies and concomitant therapeutic actions of the drug [24,26-30]. Initially formulations hold the drug into them then improve their activities via encapsulations [29]. Thus, in present study we succeed to give good formulation for enriching the antioxidant activity of Ceftriaxone through encapsulation in biocompatible emulsions formulated using neem oil and aqueous DTAB.
Materials and Methods
The commercially available neem oil was procured, and other chemicals such as DTAB, Ceftriaxone and DPPH free radical were obtained from Sigma Aldrich having 99.99% purity level. The chemicals were used as received and Milli-Q water of conductivity 10-7 Scm-1 was used.
Preparation of formulations
0.1-0.5 mL oil was separately taken into 50 mL RB flask using a pipette, then, a 50 mL 0.001 M aqueous DTAB solution was added in all of the formulations at 1000 rpm stirring. The stirring was further sustained for 45 min, under similar conditions, in each case.
Ceftriaxone encapsulated emulsions
For Ceftriaxone loading, initially, 10 uM (in methanol) was dissolved in neem oil, before the oil-drug mixture was kept for magnetic stirring at 1000 rpm, so as to enable complete solubilisation. This process of adding Ceftriaxone in neem oil has been referred to as drug encapsulation and this oil, only, has been further used to prepare drug loaded emulsions (DLEs).
Physicochemical characterizations
Densities were measured using Anton Paar DSA 5000M Density meter, with the respective accuracies being ± 10-6g cm-3 and ± 0.5 m/s, respectively. The instrument was calibrated with water and dry air (DMA, manual; Anton Paar, Graz, Austria). The viscosities and surface tensions of drug encapsulated and pure formulations were measured using Borosil Mansingh Survismeter, with the respective accuracies of ± 0.01 mN/m and ± 1 × 10-4mPa-s [31,32]. The pH and potential were checked using Lab India instruments.
Measurement of drug loading efficacy
The drug loading efficacies for Ceftriaxone were determined through the comparison of densities, surface tension, viscosities, pH and potentials of with and without drug loaded formulations. These were measured in terms of comparative % encapsulation, surface availability and binding potential using densities, surface tension and viscosities respectively as per reported by Parth Malik et al. [33].
Measurements of antioxidant potential
Spectrophotometric method: Antioxidant activities, RSAs, of the DLEs are measured by a free radical scavenging effect of steady DPPHÃÆâÃâââ¬âÃâÃÂ, with spectrophotometric titration with minor alteration [34,35]. For screening RSAs, pure DPPHÃÆâÃâââ¬âÃâàsolution was varied with DLEs in 1:1 ratio. Thereafter, on vigorous shaking, these samples were kept in dark for an incubation period of 45 min. The RSAs were assessed as a measure of comparative % reduction in absorbance of pure DPPHÃÆâÃâââ¬âÃâàand DPPH + DLE 1:1 mixture, at λmax= 517 nm. The measurements were made with Spectro 2060 plus model UV/Vis spectrophotometer. Firstly base line correction for the pure emulsion was made. The respective RSAs were quantitatively estimated using the following equation [34,35].
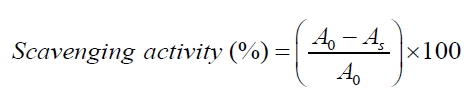 (1)
(1)
A0 and As are the absorbance of pure DPPHÃÆâÃâââ¬âÃâàand DLEs.
Physicochemical methods: First time, this attempt has been made that the RSAs are evaluated through physicochemical analysis. In this method the free radical interaction with drug in term of RSA has been determined via comparative analysis of PCPs. The overall interaction with free radicals, surface scavenging and bulk scavenging activities have been determined through the comparison of densities, surface tension and viscosities of pure DPPH and DPPH + DLEs. The mathematical equations derived for analysing these attributes are as follow.
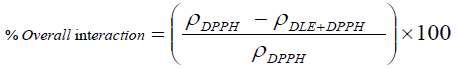 (2)
(2)
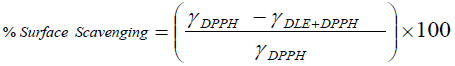 (3)
(3)
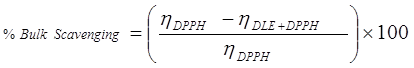 (4)
(4)
There ρ, γ and η are respective values of densities, surface tension and viscosities. The RSA are also analysed with potential studies of with and without drug encapsulated emulsions with compare to potential of DPPH. The % scavenging effect was analysed as follow.
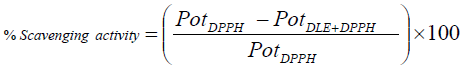 (5)
(5)
Results and Discussion
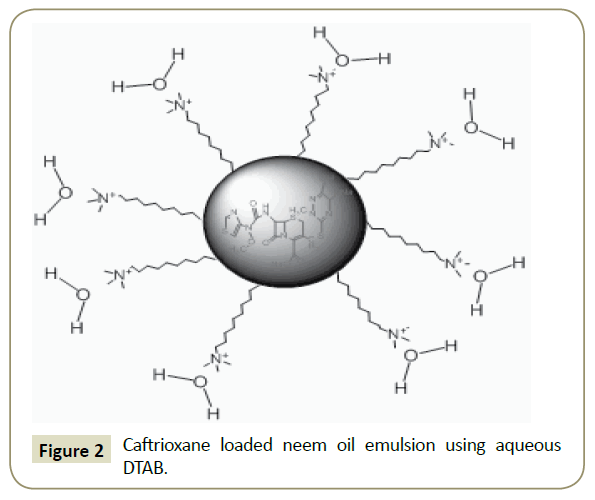
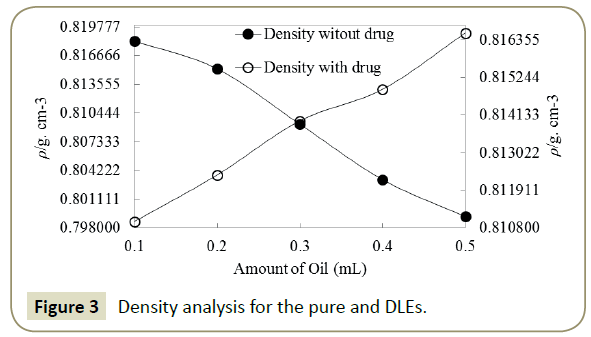
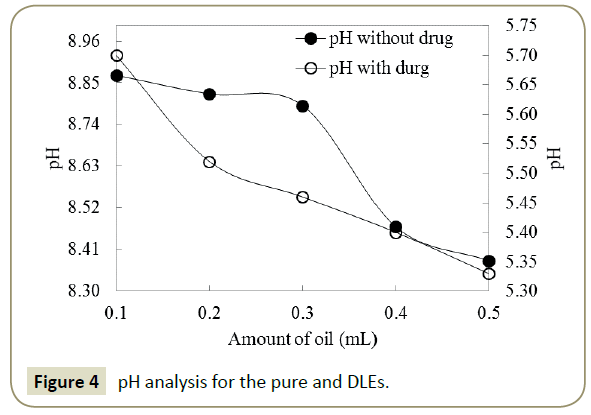
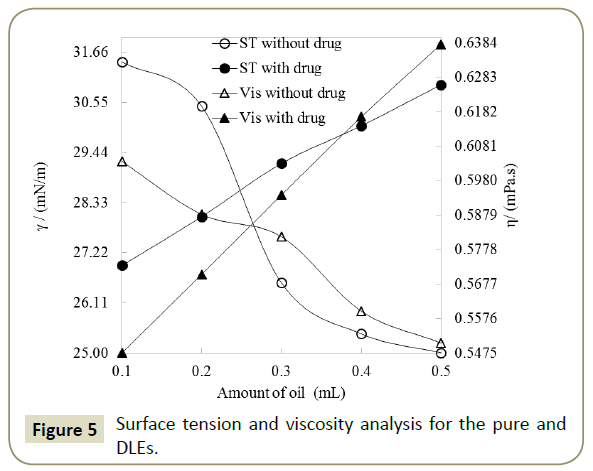
Neem Oil/ Aq. Surfactant emulsions: Physicochemical characterizations After one hour stirring of mixture of oil and aqueous DTAB produced milky coloured emulsion, predicted structure is shown in Figure 2. The pure and DLEs carrying Ceftriaxone encapsulated neem oil and aq. surfactants have been critically inspected via PCPs to comprehend and analyse the dissolution and dispersal pattern of Ceftriaxone. In understanding of this, the characteristic densities, surface tensions, viscosities, pH and potential of the formulations have been assessed. Density is a measurement of compression and is caused due to interaction of constituents of the system and able to discriminate between interrelating systems [33]. So, the density of pure and DLEs has been deliberate to differentiate between these pure and DLEs (Figure 3), where the values of densities have been found to be reduced with the amount of oil for the pure formulation. It may be due to the higher amount of the oil has increased the hydrophobicity into ternary system (Oil + Water + Surfactant) that caused weaker interaction among the components, and resulted lower in densities. In case of DLEs the densities got augmented with the amount of oil followed by reverse trend of pure formulations that confirmed the loading of the drug or DLEs. Ceftriaxone having many hydrophilic groups decreased the hydrophobic effect of the oil, and increased the interaction concluding the effectiveness of the drug with the oil. The examination of pH of pure and DLEs inferred the acidic nature of the oil which led towards acidic from basic (Figure 4). Since ceftriaxone have acidic groups therefore the pH of pure formulation higher than the pH of DLEs because drug has increased the acidic character of formulation. Especially with the 0.2 and 0.3 mL of oil the pH of DLEs decreased much as compared to other, such concentration may be noticed as effective concentration for particular formulation. Such comparative analysis of pH with respect to pure and DLEs confirmed the drug loading in emulsion where the decrease in pH for DLEs from pure formulation has been found. Similarly, the surface tension and viscosity, as interface parameters, reveal about the interaction with respect to surface and bulk respectively. Surface tension and viscosity data from 0.1 to 0.5 mL with 0.1 mL interval for pure and DLEs are calculated as per literature [31,32]. For the pure formulation both the surface tension and viscosity have been decreased while for the DLEs the same were found to be increased with respect to oil’s amount. The increase and decrease in both the parameters for the same solutions can be explained on the basis of ASIAM model [36]. The surface tension is a surface property where the molecules or particles are pulled down due to attraction of bulk causing tense at surface with a unchanging molecular preparation in liquid stage, here the surface interaction has been caused due to intermolecular interaction among the water + surfactant + oil. Data (Table 1) reveal that this interaction is weaker than pure water inferring weaker surface interaction in three component system. Initially, the oil disrupts the aqueous surfactant interaction (water + surfactant molecules) which is stronger than oil-surfactant-water interaction. On an increasing amount of oil, the surface tensions and viscosities got decreased, it infers that number of oil molecules favor a development of three components interacting system. Surface tension and viscosity with the amount of oil have varied linearly depicted in Figure 5, due to, composition effect on interactions. In the case of DLEs, the viscosity and surface tension have behaved inversely as compared to pure formulation where these have been increased with respect to amount of oil. It inferred that the ceftriaxone having more hydrophobicity strengthened the interaction of oil-surfactant-water, and confirmed the loading of the drug into the formulation. Such variations in surface tension and viscosity for the pure and DLEs furnish significant information about the emulsion system for conducting the drug delivery process.
| Components of Formulations with and without drug | |||
|---|---|---|---|
| Oil qty.(mL) | Conc. of Aq. DTAB (M) | Qty. of Aq. DTAB(mL) | Conc. of Drug (µM) |
| 0.10 | 0.001 | 50 | 10 |
| 0.20 | 0.001 | 50 | 10 |
| 0.30 | 0.001 | 50 | 10 |
| 0.40 | 0.001 | 50 | 10 |
| 0.50 | 0.001 | 50 | 10 |
Table 1: Reports components of formulations with and without drug.
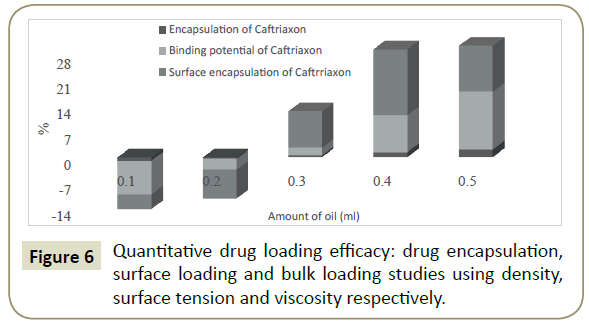
Quantitative drug loading efficacy (QDLE)
As drug is loaded into any system it is distributed homogeneously in the solution, and this distribution is analyzed in term of QDLE using equations reported by Parth et al. [33] where drug encapsulation, surface loading and bulk loading have been studied using density, surface tension and viscosity respectively. Figure 5 shows an analysis of QDLE where increment and decrement have been observed in binding potential, surface encapsulation and total encapsulation (-ve sign reflects decrease in respective value). With the increasing amount of oil the increase in surface encapsulation was found to be increased till 0.4 mL, which may be due to that, till this concentration surface activity is maximum, and after that the same was found to be decreased. Ceftriaxone empathies, relative encapsulation efficacy and doings with oil and surfactants were assessed for relative changes in PCPs (Table 2) [37,38]. Hence, the encapsulation efficiency of Ceftriaxone was appraised by the analysis of comparative changes in densities (ρ), with Eq. (1) quantitatively. The % encapsulation is illustrated in Figure 6, where a negative sign represents that drug works as structure breaker while a positive sign shows it as a structure maker [36]. The higher densities of ceftriaxone with oil and aqueous DTAB solutions reflect that the Ceftriaxone interrelated strongly with the components of systems. Since the densities of drug loaded formulations with the surfactants were found less than those of reliable aqueous surfactant solutions (Table 2 and Figure 3). These experimental trends infer that the surfactants probably industrialised micelles and captured Ceftriaxone altogether. A maximum drug encapsulation (2.27%) has been reached at 0.5 mL of oil. Similarly, surface tension is a surface property, reducing the surface molecules to improvement high energy as a result of being surrounded with fewer neighbours, thereby, the surface tension variations of DLEs with those of pure formulations enable a quantifiable estimation of surface availability of the ceftriaxone has been calculated [39]. These values seemed as numerically negative with the amount of 0.1 and 0.2 mL of oil, whereas for 0.3, 0.4 and 0.5 mL, the corresponding values were found to be positive. A positive sign for the characteristic surface availability tells that the ceftriaxone is separated out of emulsion components and gradually obtains a concentrative existence at the surface. This accomplishes that the introduction of drug reorients the emulsion in a way that the oppositely charged poles aggregate on the ceftriaxone as a core and stronger binding forces are developed that improved the surface tension values in DLEs. It can be further experienced through the RSAs, which are higher. A maximum comparative increase of 23.76% is attained in surface tension at 0.5 mL of oil. From such differences, it is very much seeming that the interactions in DLEs are highly subtle and thereby moderate the surface tension of the ternary mixtures so as to bring the saturated drug molecules over to the surface. The physical and dimensional sequences and factors of constituents of ternary systems result in the development of a recognised box encircling ceftriaxone to not only bind but also to facilitate its transportation. In contrast of surface tension values of pure and DLEs, incidental surface tensions decrease upon the drug encapsulation, with a stronger dispersion of drug. The viscosities (η) of DLEs enable the approximation of intermolecular forces (IMFs) [40] so, a quantitative estimate of corresponding inter conversion of cohesive forces into IMFs, has been made, through a regular interaction of the structural areas. An increase in viscosities shows the broken of cohesive forces occupied among the solute molecules with the slow development of IMFs, after an addition of drug. Hence, the selected surfactant catalysis, as perfect environment for refining the antioxidant is the resultant therapeutic efficiency of the drug. This is so as in all these DLEs, the viscosities get increased in process overlap way for the breaking of cohesive forces within the interaction scenery and their corresponding gradual replacement by IMFs between the ceftriaxone and surfactant. It is imitated by comparative encapsulation values of the ceftriaxone within the interaction medium that also emphases the drug acting as a structure breaker in such DLEs, irrespective of the working quantity of oil.
| Qty oil (mL) | pH | V | ρ/ ± 10-6g.cm-3 | η/ ± 10-4mPa.s | γ / ± 10-2mN/m |
|---|---|---|---|---|---|
| Without drug | |||||
| H2O | 7.20 | 0.120 | 0.994034 | 0.7225 | 70.38 |
| DTAB | 7.40 | 0.063 | 0.807271 | 0.5780 | 40.98 |
| 0.1 | 8.87 | 0.030 | 0.818256 | 0.6036 | 31.45 |
| 0.2 | 8.82 | 0.027 | 0.815243 | 0.5881 | 30.46 |
| 0.3 | 8.79 | 0.026 | 0.809232 | 0.5816 | 26.55 |
| 0.4 | 8.47 | 0.026 | 0.803222 | 0.5598 | 25.42 |
| 0.5 | 8.38 | 0.022 | 0.799211 | 0.5504 | 25.00 |
| DLEs | |||||
| 0.1 | 5.70 | 0.136 | 0.810989 | 0.5475 | 26.94 |
| 0.2 | 5.52 | 0.132 | 0.812365 | 0.5706 | 28.02 |
| 0.3 | 5.46 | 0.128 | 0.813965 | 0.5938 | 29.20 |
| 0.4 | 5.40 | 0.125 | 0.814896 | 0.6167 | 30.03 |
| 0.5 | 5.33 | 0.116 | 0.816562 | 0.6380 | 30.94 |
| DPPH + DLEs | |||||
| DPPH | 5.99 | 0.21 | 0.793569 | 0.6546 | 43.57 |
| 0.1 | 5.42 | 0.164 | 0.822256 | 0.6491 | 33.52 |
| 0.2 | 5.45 | 0.147 | 0.821246 | 0.6103 | 36.22 |
| 0.3 | 5.65 | 0.144 | 0.819336 | 0.6044 | 37.36 |
| 0.4 | 6.18 | 0.136 | 0.815148 | 0.5880 | 38.48 |
| 0.5 | 6.46 | 0.123 | 0.812258 | 0.5793 | 40.47 |
Table 2: Experimental pH, potential (V), density (ρ), viscosity (η), and surface tension (γ) of pure emulsion (without drug), Drug Loaded Emulsion (DLEs), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and DPPH + DLEs at T= 303.15 K.
Investigation of enhanced antioxidant potencies through DPPHÃÆâÃâââ¬âÃâàassay
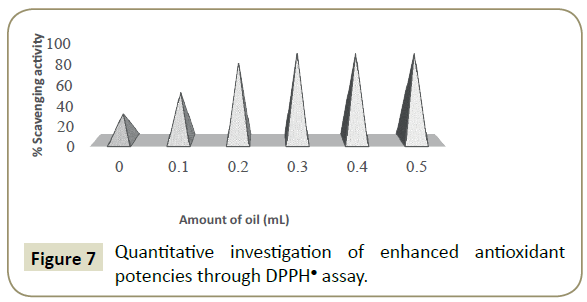
DPPHÃÆâÃâââ¬âÃâàis an odd electron radical, owning a strong characteristic UV/Vis optical density at 517 nm, due to the availability of empty space [41,42]. This nature lets the DPPHÃÆâÃâââ¬âÃâàto produce a violet colour in solution [42]. However, it develops colourless on being neutralized on for aging of its electron by H+, made obtainable through free radical scavenging antioxidant. The DPPHÃÆâÃâââ¬âÃâàshows a paramagnetic nature, mainly responsible for showing a characteristic specific EPR signal [36]. Due to this, DPPHÃÆâÃâââ¬âÃâàactivity assessment is used to approximation RSAs of DLEs through measurement of relative% decrease in absorbance at 517 nm, in accordance with Eq. (1). The corresponding % scavenging activities have been represented in Figure 7. For study of a critical inspiration of emulsions mediated antioxidant activity, the RSAs of pure ceftriaxone were also assessed separately. The interactions of antioxidants with DPPHÃÆâÃâââ¬âÃâàproceed at mutable rates and relative kinetics, depending on relative ease of antioxidant action and a corresponding effect of UV light [43,44]. A decrease in absorbance infers different modes of antioxidant activity, thus, a particular antioxidant causing a reduction of initial DPPH absorbance. Figure 7 illustrates analysis for free RSAs of prepared DLEs, where pure ceftriaxone has exposed nearly 23% RSAs respectively, while upon loading it has exposed maximum 86% with respect to amount of oil. So, an improvement in the antioxidant aptitude of Ceftriaxone was observed when it was abridged within emulsions. DTAB having three methyl group which results in the stearic interference with hydrophilic portion, eventually resulting in stronger hydrophobic interactions, leading to a formation of larger aggregates in the formulation since the ceftriaxone with oil leads to the arrival of additive hydrophobicity in the medium. Further, it was also experimental that an optimal concentration effect with respect to loading effectiveness and activity of ceftriaxone seems to play an important role in enhancing its medicinal efficacy. As the effect of ceftriaxone’s antioxidant effectiveness through emulsions was deliberate without temperature fixation, this leads to a dependable speculation that the emulsions possess an oil content dependent RSAs which typically restrains the interaction and corresponding free radical overload potential (Figure 7).
Investigation of antioxidant potencies through physicochemical properties
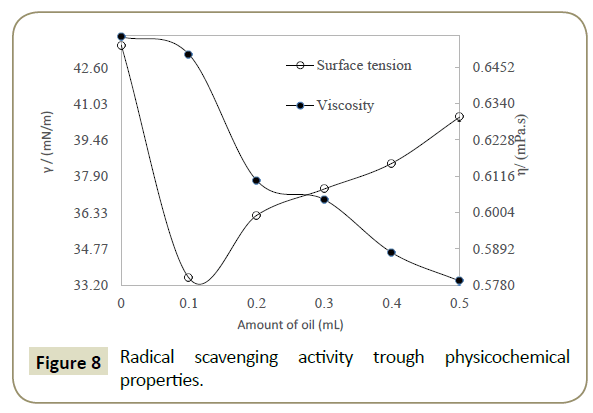
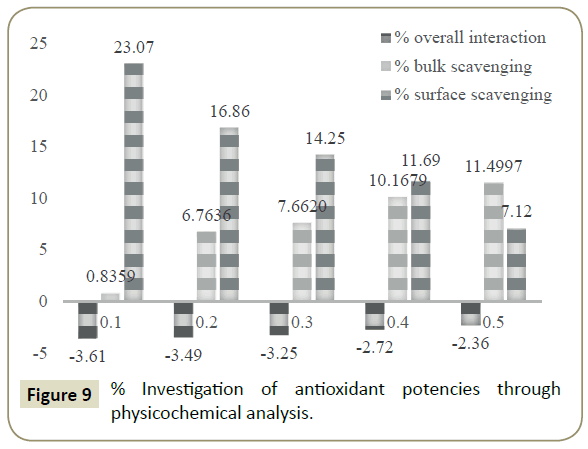
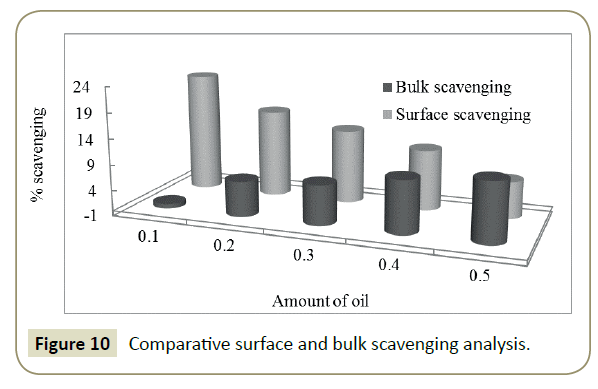
The antioxidant potential study or interaction with DPPH free radical has also been examined through measuring the difference in the data of PCPs. Similar to spectrophotometric method, the decrease in values of surface tension and viscosity of DPPH+DLEs from pure DPPH was made a basis for RSAs. The viscosity and surface tension of pure DPPH solution were found 0.6546 Ps and 43.57 mN. m respectively, when the same values were compared with the values of DLEs + DPPH solutions then, these were found to be decreased as shown in Figure 8. With the analysis of surface tension the same values has been decreased only for 0.1 mL oil and afterward these have been increased inferring the effect of concentration of oil. Analysis of viscosity revealed that these have been continues decreased with the increasing amount of oil inferring better physicochemical tool for analysis of RSA. The % difference in density, surface tension and viscosity of DPPH and DPPH + DLEs were calculated as per equations 2, 3 and 4, these are referred as overall interaction, surface scavenging and bulk scavenging respectively, shown in Figure 9. The –ve values for overall interaction show strong interaction which is increased with respect to oil amount. The % comparative analysis of bulk and surface scavenging is shown in Figure 10 where they are showing reverse trend infers that as surface scavenging is decreased the bulk scavenging is increased with the amount of oil. Initially surface scavenging is high because at first the interaction occurs at surface then slowly interacts in bulk.
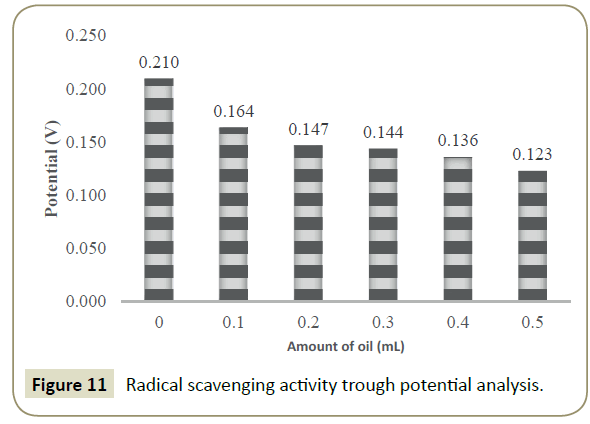
Investigation of antioxidant potencies through potential analysis
Similarly the RSA is also analyzed with potential study as mentioned in equation 5, initially the potential of pure DPPH was taken and then it was compared with the potential of DPPH + DLEs. With respect to spectrophotometry, the decrease in potential of DPPH was made criteria for the RSA analysis. Potential of pure DPPH was 0.210 V, and decrease in the same was noticed by the addition of DLEs as shown in Figure 11, where zero shows potential of pure DPPH.
Conclusion
Our study thus proves that the encapsulation of ceftriaxone within the physically active formulations leads to the optimization of its activity on an in-vitro basis. The formulated emulsions to supplement the immune protective attributes of ceftriaxone, that otherwise remain impaired due to its rapid clearance from the systemic tissue environment. The investigation of the ceftriaxone loaded formulations using physicochemical accounts for the fact that, in general, with an increase in oil, the corresponding parameter goes on increasing. Also, the physicochemical trends reflect the fact that an optimum of oil and surfactant combination is essential for making the emulsions as ideally feasible with a viewpoint to augment the antioxidant efficacy of ceftriaxone, due to the existence of a canonical ensemble. The incorporation of neem oil in current study is also evidently justified through the symmetric distribution and interaction patterns of PCPs. Even though improved free radical scavenging activity is obtained with such formulations, further, there is a sharp inflection and optimization of physicochemical features of formulations, leading to the best efficiency towards an enhanced antioxidant effect. The reliable and promising outcomes of this study have been taken in due spirit, where in absence of DPPH the antioxidant analysis could be carried out with physicochemical properties. The radical scavenging activities (RSA) of the encapsulated Ceftriaxone, have thus, confirmed an improvement in its antioxidant efficacy and the analysis of PCPs has depicted its loading efficacy and relative binding ability. Therefore, our study could not only furnish better understanding of enhancing the RSA of Ceftriaxone but also lead to the design of novel formulations with other surfactants and co-surfactants, thereby enabling better stability.
Acknowledgment
Authors are thankful to Kadi Sarva Vishwavidhyalaya, Gandhinagar, Gujarat for infrastructural support and experimental facilities.
References
- Su T, Mulla MS (1998) Ovicidal activity of neem products (azadirachtin) against Culex tarsalisand Culex quinquefasciatus (Diptera: Culicidae). J Am Mosq Control Assoc 14: 204-209.
- Sukumar K, Perich MJ, Boobar LR (1991) Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc 7: 210-237.
- Dhar R, Dawar H, Garg S, Basir SF, Talwar GP (1996) Effect of volatiles from neem and other natural products on gonotrophic cycle and oviposition of Anopheles stephensiand An. Culicifacies (Diptera: Culicidae). J Med Entomol 33: 195-201.
- Su T, Mulla MS (1999) Effects of neem products containing azadirachtin on blood feeding, fecundity and survivorship of Culextarsalis and Culex quinquefasciatu (Diptera: Culicidae). J Vector Ecol 24: 202-15.
- Lucantoni L, Giusti F, Cristofaro M, Pasqualini L, Esposito F, et al. (2006) Effects of neem extract on blood feeding, oviposition and oocyte ultrastructure in Anopheles stephensi Liston (Diptera: Culicidae). Tissue Cell 38: 361-71.
- Mulla MS, Su T (1999) Activity and biological effects of neem products against arthropods of medical and veterinary importance. J Am Mosq Control Assoc 15: 133-52.
- Boschitz C, Grunewald J (1994) The effect of NeemAzal on Aedes aegypti (Diptera: Culicidae). Appl Parasitol 35: 251-6.
- Budavari S (2001) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals 13th edition, White House Station, NJ, USA.
- Tariq A, Siddiqui MR, Kumar J, Reddy D, Negi PS, et al (2010) Development and vali- dation of high performance liquid chromatographic method for the simultaneous determination of ceftriaxone and vancomycin pharmaceutical formulations and biological samples. Science Asia 36: 297-304.
- Shah J, Jan MR, Shah S, Khan MN (2013) “Development and validation of HPLC method for simultaneous determination of ceftriaxone and cefaclor in commercial formulations and biological samples. J Mex Chem Soc 57: 314-320.
- Sun H, Wang H, GE X, Quin X (2011) Simultaneous deter- mination of the combined drugs ribavarin and ceftriaxone sodium in human urine by HPLC-DAD. IJSID 1: 216-225.
- Kale RS, Jain HK, Ghode PD, Mhaske GS, Puri MV, et al. (2011) An RPHPLC method for simultaneous estimation of Ceftriaxone sodium and sulbactam sodium in parenteral dosage form. Int J Pharm Pharm Sci 3: 406–409.
- Kumar RN, Rao GN, Naidu PY (2010) Stability indicating fast LC method for determination of ceftriaxone and tazobactam for injection related substances in bulk and pharmaceutical formulation. Int J Appl Biol Pharm 1: 145–157.
- Shrivastava SM, Singh R, Tariq A, Siddiqui MR, Yadav J, et al. (2009) A novel high performance liquid chromatographic method for simultaneous determination of ceftriaxone and sulbactam in sulbactomax. Int J Biomed Sci 5: 37–43.
- Nirav BP, Alpesh CA, Sneha KJ, Vipul DM, Hemant TD (2012) Development and validation of stability indicating method for simultaneous estimation of ceftriaxone and sul- bactam injection using RP-UPLC method. IOSR J Pharm 2: 29–37.
- Jat RK, Chhipa RC, Sharma S (2010) Spectrophotometric Quantification of Etoricoxib in Bulk Drug and Tablets Using Hydrotropic Agent. Pharmacophore 1: 96-102.
- Jat RK, Chhipa RC, Sharma S (2010) Spectrophotometric quantification of carvedilol in bulk drug and tablets. Pharmacophore 1: 90–95.
- Jat RK, Chhipa RC, Sharma S (2010) Quantification of clobazam in bulk drug and tablets. Int J Pharm Sci Rev Res 1: 18–24.
- Jat RK, Chhipa RC, Sharma S (2011) Spectrophotometric estimation of fluvoxamine maleate in tablets using hydrotropic agent. Intern J Pharmac Quality Assurance 2: 73-75.
- Jat RK, Sharma S, Chhipa RC (2011) Spectrophotometric and HPLC methods for Poorly Water Soluble Drugs. Ph.D. thesis, SGVU Jaipur, Rajasthan 166-170.
- Pal N, Rao AS, Hedi MA (2001) HPLC method development and validation for the assay of ceftriaxone sodium injection. J Pharm Pharm Sci 2: 84-90.
- Merck & Co. (2007) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals 14th Edn Whitehouse Station, NJ, USA.
- Subhashis D, Satayanarayana, Gampa VK (2010) Nanoemulsion-a method to improve the solubility of lipophilic drugs. Int. J. rev. Pharm 3: 0541-2231.
- Thakur, MK Walia, S.L.H (2013) Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: A Review. Pharmacophore 4: 15-25.
- McClements DJ, Rao J (2011) Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr 51: 285-330.
- Mason TG, Wilking JN, Meleson, Chang CB, Graves SM (2006) Nanoemulsions: formation, structure, and physical properties. J Phys Condens. Matter 18: 635–666.
- Gibaud S Attivi D (2012) Microemulsion for oral administration and their therapeutics applications. Expert Opin Drug Deliv 9: 937-951.
- Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H (2012) Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J Nanotechnol 1-12.
- Afornali A, Vecchi R, Stuart RM, Dieamant G, Lima de L, et al. (2013) Triple nanoemulsion potentiates the effects of topical treatments with microencapsulated retinol and modulatesbiological processes related to skin aging. An Bras Dermatol 88: 930-936.
- Hamoudah SYM, Hassanin MM, Youssef GA (2010) Protective role of ginger and curcumin against some toxicological effects induced by thermoxidized frying cotton oil. Com Sci 1: 35-42.
- Singh M (2006) Survismeter — Type I and II for surface tension, viscosity measurements of liquids for academic and research and development studies. J Biochem Biophys Methods 67: 151–161.
- Singh M (2007) Survismeter, 3-in-1 Instrument for Simultaneous Measurements of Surface Tension, Inter Facial Tension (IFT) and Viscosity. Pak J Anal Environ Chem 8: 82-85.
- Malik P, Ameta RK, Singh M (2014) Preparation and characterization of bionanoemulsions for improving and modulating the antioxidant efficacy of natural phenolic antioxidant curcumin. Chem Biol Interact 222: 77–86.
- Patel Rajesh M, Patel Natvar J (2011) In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. JAPER 1: 52-68.
- Enujiugha VN, Talabi JY, Malomo SA, Olagunju AI (2012) DPPH Radical Scavenging Capacity of Phenolic Extracts from African Yam Bean (Sphenostylisstenocarpa). Food Nutr Sci 3: 7-13.
- Ameta RK, Singh M (2015) Surface tension, viscosity, apparent molal volume, activation viscous flow energy and entropic changes of water+ alkali metal phosphates at T = (298.15, 303.15, 308.15). J Mol Liq 203: 29–38.
- McClements DJ (2012) Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8: 1719-1729.
- Mayer S, Weiss J, McClements DJ (2013) Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: Factors influencing droplet size and stability. J Colloid Interface Sci 402: 122–130.
- Soni KB, Kuttan R (1992) Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Ind J Physiol Pharmacol 36: 273–275.
- Ostertag F, Weiss J, McClements DJ (2012) Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J Colloid Interface Sci 388: 95–102.
- Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK (2007) Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330: 155–163.
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, et al (2007) Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 60: 171–177.
- Kitawat BS, Singh M, Kale RK (2013) Solvent free synthesis, characterization, anticancer, antibacterial, antifungal, antioxidant and SAR studies of novel (E)-3-aryl-1-(3-alkyl-2-pyrazinyl)-2-propenone. New J Chem 37: 2541-2550.
- Ameta RK, Singh M, Kale RK (2013) Synthesis and structure–activity relationship of benzylamine supported platinum (IV) complexes. New J Chem 37: 1501-1508.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences