Protonation of Nitrofurantoin and Furazidine Molecules in Acidic MediaMolecular Modelling Studies
Somogi A*, Dabrowska A, Ziemba R and Pawinski T
DOI10.21767/2469-6692.10025
Somogi A1*, Dąbrowska A2, Ziemba R3 and Pawiński T1
1Department of Drug Chemistry, Medical University of Warsaw, 1 Banacha Str., 02- 097 Warsaw, Poland
2Departments of Cell Biology, National Medicines Institute, 30/34 Chełmska Str., 00-725 Warsaw, Poland
3Lux Med Group - Medical Center, 51 Prosta Str., 00-838 Warsaw, Poland
- *Corresponding Author:
- Aleksander Somogi
Department of Drug Chemistry
Medical University of Warsaw
1 Banacha Str., 02-097 Warsaw, Poland
E-mail: aleksander.somogi@wum.edu.pl
Received Date: September 19, 2018; Accepted Date: October 08, 2018; Published Date: October 15, 2018
Citation: Somogi A, Dąbrowska A, Ziemba R, Pawiński T (2018) Protonation of Nitrofurantoin and Furazidine Molecules in Acidic Media-Molecular Modelling Studies. J In Silico In Vitro Pharmacol 4:3. DOI: 10.21767/2469-6692.10025
Abstract
The molecular modeling studies on protonation sites of Nitrofurantoin and Furazidine as well as on the stability of particular protonated forms were performed using quantum chemical MP2 method. Performed calculations show that Furazidine oxygen and nitrogen atoms are better proton acceptors than in Nitrofurantoin, therefore the acidity of the media may differentiate Nitrofurantoin and Furazidine antibacterial activity.
Keywords
Nitrofurantoin; Furazidine; Acidity; Protonation sites; Urinary tract infection; Molecular modelling
Introduction
According to literature data in nitrofuran derivatives the major role in antimicrobial activity plays the acidity of physiological medium in urinary tract [1]. It was pointed out [2] that dissociation of Furazidine is hampered in the presence of ascorbic acid (vitamin C). The role of any acidifying agent like ascorbic acid is to prevent alkalization of infected urine and preserve pH close to 5.5 in which Furazidine molecule stays in non-dissociated form what enhances its antibacterial activity [1]. More acidic medium can probably cause better protonation of the Furazidine molecule than of Nitrofurantoin, what can further enhance Furazidine therapeutic efficacy. Therefore studies of possible protonation sites of Nitrofurantoin and Furazidine, along with stability of particular protonated form, can provide valuable estimate for insight into activity determinates of Furazidine moiety, and also for further modification of its structure. Selection of Nitrofurantoin and Furazidine is a wise model because those two compounds differ in two carbon atoms in the rings bridging part of molecules. Moreover there is distinct difference between their antimicrobial activities. In Escherichia coli test they display MIC ≤ 32 μg/ml and 1 μg/ml for Nitrofurantoin and Furazidine, respectively [3], as reported in information leaflets of medicine present on the market.
Methods
All quantum chemical calculations were performed with Spartan 14 V.1.1.4 software package at the MP2/6-31G*//MP2/6-31G* level [4]. The hydration energies were calculated with SM8 model.
Results and Discussion
Calculation on neutral Nitrofurantoin tautomer clearly indicates that N1-H tautomer is the most stable form both in water medium. The same holds for Furazidine molecule.
The neutral Nitrofurantoin and Furazidine molecules can potentially exist in various tautomeric forms. As shown in Tables 1 and 2 the N1-H tautomer of Nitrofurantoin is the most stable.
| Neutral | Total energy | Stabilization energy | ΔG0 | Δ (ΔG0) |
|---|---|---|---|---|
| I (N1-H) | -900.956670 | 0.0 | -900.823127 | 0.00 |
| II (N3-H) | -900.919856 | 23.10 | -900.777778 | 28.46 |
| III (O1-H) | -900.928299 | 17.80 | -900.777699 | 28.51 |
| IV (O2-H) | -900.928428 | 17.72 | -900.786946 | 22.70 |
| V (N2-H) | -900.936229 | 12.83 | -900.793088 | 18.85 |
| VI (O3-H) | -900.869400 | 54.76 | -900.745721 | 48.57 |
| VII (O4-H) | -900.873576 | 52.14 | -900.750943 | 48.57 |
| Cation | ||||
| I (N1-H; O1-H) | -901.208345 | -157.93 | -901.027358 | -128.15 |
| II (N1-H; O2-H) | -901.369024 | -258.75 | -901.132061 | -193.86 |
| III (N1-H; O3-H) | -901.383507 | -267.84 | -901.150271 | -205.28 |
| IV (N1-H; N1-H) | -901.336768 | -238.51 | -901.116485 | -184.08 |
| V (N1-H; N2-H) | -901.364274 | -255.77 | -901.129443 | -192.21 |
| VI (N1-H; N3-H) | -901.383523 | -267.85 | -901.150258 | -205.28 |
| VII (N1-H; O4-H) | -901.355919 | -250.53 | -901.135881 | -196.25 |
| VIII (N3-H; O4-H) | -901.315992 | -225.47 | -901.093608 | -169.73 |
| Dication | ||||
| I (N1-H; N1-H; N3-H) | -901.763484 | -506.28 | -901.304039 | -301.77 |
| II (N1-H; O1-H; N3-H) | -901.785163 | -519.88 | -901.328894 | -317.37 |
| III (N1-H; O2-H; N3-H) | -901.784169 | -519.26 | -901.329481 | -317.74 |
| IV (N1-H; N1-H; O2-H) | -901.733673 | -487.57 | -901.289431 | -292.61 |
| V (N1-H; O1-H; O2-H) | -901.767780 | -508.97 | -901.289982 | -292.95 |
| VI (N1-H; N1-H; N2-H | -901.727780 | -483.87 | -901.246460 | -265.64 |
| VII (N1-H; N2-H; N3-H) | -901.762048 | -505.37 | -901.296180 | -296.84 |
| VIII (N1-H; N2-H; O1-H) | -901.482729 | -330.10 | -901.220942 | -249.63 |
| IX (N1-H; N2-H; O2-H) | -901.734154 | -487.87 | -901.254361 | -270.60 |
Table 1: Total MP2/6-31G*//6-31G* energies, stabilization energies, ΔG0 and Δ(ΔG0) calculated for neutral and protonated Nitrofurantoin molecule.
| Neutral | Total energy | Stabilization energy | ΔG0 | Δ(ΔG0) |
|---|---|---|---|---|
| I (N1-H) | -978.101505 | 0.00 | -977.934728 | 0.00 |
| II (N3-H) | -978.047774 | 33.72 | -977.883044 | 32.43 |
| III (O1-H) | -978.079351 | 13.90 | -977.896975 | 23.69 |
| IV (O2-H) | -978.073821 | 17.37 | -977.898060 | 23.01 |
| V (N2-H) | -978.065152 | 22.81 | -977.903567 | 19.55 |
| VI (O3-H) | -978.011941 | 56.20 | -977.856693 | 48.97 |
| VII (O4-H) | -978.011940 | 56.20 | -977.856734 | 48.94 |
| Cation | ||||
| I (N1-H; O1-H) | -978.497706 | -248.62 | -978.236155 | -118.53 |
| II (N1-H; O2-H) | -978.501750 | -251.15 | -978.252367 | -199.32 |
| III (N1-H; O3-H) | -978.501737 | -251.15 | -978.252361 | -199.31 |
| IV (N1-H; N1-H) | -978.483563 | -239.74 | -978.230392 | -185.53 |
| V (N1-H; N2-H) | -978.510679 | -256.76 | -978.250115 | -198.55 |
| VI (N1-H; N3-H) | -978.538595 | -274.27 | -978.274167 | -213.00 |
| VII (N1-H; O4-H) | -978.499000 | -249.43 | -978.249830 | -197.73 |
| VIII (N3-H; O4-H) | -978.460211 | -225.09 | -978.215781 | -176.36 |
| Dication | ||||
| I (N1-H; N1-H; N3-H) | -978.913382 | -509.45 | -978.438447 | -316.08 |
| II (N1-H; O1-H; N3-H) | -978.940486 | -526.46 | -978.466285 | -333.55 |
| III (N1-H; O2-H; N3-H) | -978.937012 | -524,28 | -978.463007 | -331.50 |
| IV (N1-H; N1-H; O2-H) | -978.878684 | -487.68 | -978.434441 | -313.57 |
| V (N1-H; O1-H; O2-H) | -978.899957 | -501.03 | -978.403282 | -294.02 |
| VI (N1-H; N1-H; N2-H | -978.866915 | -480.29 | -978.370630 | -273.53 |
| VII (N1-H; N2-H; N3-H) | -978.914768 | -510.32 | -978.431724 | -311.86 |
| VIII (N1-H; N2-H; O1-H) | -978.894982 | -497.91 | -978.395595 | -289.19 |
| VIII (N1-H; N2-H; O2-H) | -978.874654 | -485.15 | -978.380531 | -279.94 |
Table 2: Total MP2/6-31G*//6-31G* energies, stabilization energies, ΔG0 and Δ (ΔG0) calculated for neutral and protonated Furazidine molecule.
The second most stable tautomer is the one bearing the proton on the O2 nitrogen atom at the MP2/6-31G*//MP2/6-31G* level with hydration energy included Table 1. Calculation of Δ (ΔGo) yields also the O2-H tautomer to be the most stable form after the N1-H tautomer (Table 2). The N3-H tautomer probably does not exist. The attached proton is being transferred from N3 to O2 atom during optimization process yielding the O2-H tautomer.
When proton is placed on O3 oxygen atom then it relocates to one of the atoms of the nitro group. The relative stability of Nitrofurantoin tautomers in water medium is as: N1-H>O2-H>O1- H>O4-H form. The same trend is observed when the Δ (ΔGo) values are considered.
In the case of Furazidine molecule also the N1-H tautomer appeared to be the most stable. Here however the N2-H and N3-H tautomers exist. The N3-H tautomer is stabilized through C-H interaction of more flexible bridging chain with O2 atom of five membered rings. As in Nitrofurantoin, when proton is placed on O3 oxygen atom then it relocates to one of the atoms of the nitro group.
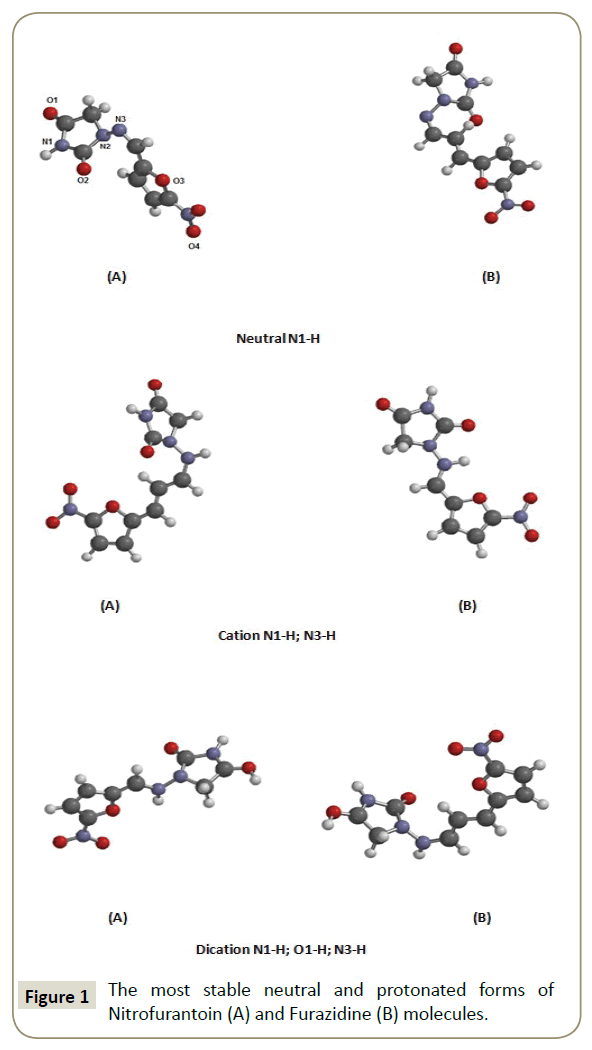
In Nitrofurantoin and Furazidine molecules there is 8 potential protonation centers, 3 oxygen atoms and 3 nitrogen atoms and two oxygen atoms of the nitro group (Figure 1). Nevertheless the nitro group, in each of two equivalent resonance structures, can potentially interact via the hydrogen bonding.
Protonation of neutral form yields mostly the other than H-N1-H+ cations what prevents the change of charge distribution and electrostatic potential pattern around non ionized fragment of neutral molecule believed to be necessary for Furazidine activity.
For Nitrofurantoin molecule the most stable is the N1-H; N3-H cation. The N1-H; O3-H cation rearranges also to that tautomer (Figure 1). For Furazidine molecule cation N1-H O3-H does not exist because it rearranges to N1-H; O2-H tautomer. The most stable like in Nitrofurantoin stays the N1-H; N3-H form Table 2.
The dication of Nitrofurantoin with the highest stability is the one formed from the most stable N1-H; N3-H cation by protonation of O1 or O2 oxygen atom. The same holds for Furazidine molecule (Table 1).
Formation of the most stable monocation N1-H N3-H is more preferred in Furazidine then in Nitrofurantoin by ca. 7.75 kcal/ mole. The most stable dication of Nitrofurantoin is N1-H; O2-H; N3- H, however very close in energy to N1-H; O1-H; N3-H. In Furazidine molecule formation of intramolecular hydrogen bonding yields the N1-H; O2-H; N3-H dication the most stable. This is due to the higher flexibility of the bridging chain, than in Nitrofurantoin.
The nitro group can be protonated at each of the oxygen atoms yielding resonance structure -NOOH+ similar to -COOH [4]. Further protonation of -NO2 could lead to reduction of –NO2 yielding the -NH2 derivative. This is one of the mechanisms that activate Furazidine active substance in the living organism [5,6].
If additional proton is placed on O3 oxygen of five membered ring of neutral Furazidine, then it relocates to O2 oxygen atom. It means that formation of cation III is very unlikely. Cation II gets additional stabilization due to intramolecular bonding with one of the oxygen atoms of -NO2 group. The most stable cation is the N-1H, N3-H. The total energy and thermodynamics (ΔG) analysis leads to the same conclusions.
Similar conclusions regarding possible protonation sites appear from analysis of electron charges on the atoms that are eager to accept proton (Table 3).
| Atomic Charges | ||||||
|---|---|---|---|---|---|---|
| Nitrofurantoin | ||||||
| Neutral N1-H Cation N1-H; N3-H Cation N1-H; N3-H |
||||||
| Atom | Electrostatic | Mulliken | Natural | Electrostatic | Mulliken | Natural |
| N1 | -0.718 | -0.731 | -0.701 | -0.544 | -0.737 | -0.689 |
| N2 | -0.009 | -0.444 | -0.375 | -0.229 | -0.371 | -0.310 |
| O1 | -0.483 | -0.447 | -0.555 | -0.404 | -0.419 | -0.527 |
| O2 | -0.461 | -0.428 | -0.529 | -0.377 | -0.356 | -0.458 |
| N3 | -0.314 | -0.195 | -0.237 | +0.215 | -0.340 | -0.228 |
| O3 | -0.237 | -0.441 | -0.401 | -0.210 | -0.462 | -0.416 |
| N4 | +0.676 | +0.276 | +0.437 | +0.631 | +0.281 +0.426 | +0.426 |
| O4 | -0.356 | -0.330 | -0.327 | -0.310 | -0.298 | -0.295 |
| O5 | -0.389 | -0.342 | -0.345 | -0.318 | -0.289 | -0.291 |
| Furazidine | ||||||
| Neutral N1-H Cation N1-H; N3-H Cation N1-H; N3-H |
||||||
| Atom | Electrostatic | Mulliken | Natural | Electrostatic | Mulliken | Natural |
| N1 | -0.663 | -0.731 | -0.699 | -0.617 | -0.728 | -0.695 |
| N2 | +0.013 | -0.439 | -0.369 | -0.132 | -0.422 | -0.372 |
| O1 | -0.459 | -0.459 | -0.568 | -0.429 | -0.418 | -0.530 |
| O2 | -0.451 | -0.433 | -0.533 | -0.401 | -0.372 | -0.474 |
| N3 | -0.347 | -0.220 | -0.246 | -0.026 | -0.379 | -0.305 |
| O32 | -0.265 | -0.405 | -0.405 | -0.202 | -0.438 | -0.401 |
| N4 | +0.681 | +0.275 | +0.437 | +0.653 | +0.279 | +0.427 |
| O4 -0.362 | -0.362 | -0.334 | -0.332 | -0.328 | -0.307 | -0.304 |
| O5 -0.393 | -0.393 | -0.346 | -0.349 | -0.335 | -0.298 | -0.300 |
Table 3: Electrostatic, Mulliken and natural charges calculated for nitrofurantoin and furazidine at MP2/631G*//MP2/6-31G* level.
For neutral Nitrofurantoin and Furazidine nitrogen atoms the most negative is the N3 atom of the bridging chain. Protonation at this atom leads to the most stable N1-H; N3-H cationic form. In the cation the most negatively charge atoms yield the most stable N1- H; O1-H; N3-H dicationic form of Nitrofurantoin, and N1-H; O1-H; N3-H dicationic form of Furazidine, respectively.
Consequently the N1-H tautomer of neutral Furazidine is more stable than N3-H, O1-H and O2-H tautomers. Performed calculations show that Furazidine oxygen and nitrogen atoms are good proton acceptors. Therefore it is justified to supplement Furazidine treatment with weak acids, for instance vitamin C, to keep the protonated Furazidine at satisfactory level, preventing urine alkalization. The in vitro studies on Furazidine acidity as function of pH are underway.
References
- Katzung BG (2015) Basic and Clinical Pharmacology. Lange Medical Books/McGraw-Hill.
- Axelrod DL (1985) Ascorbic Acid and Urinary pH. JAMA 254: 1310-1311.
- Mannisto P, Karttunen P (1979) Pharmacokinetics of furagin, a new Nitrofurantoin congener on human volunteers. Int J Pharmacol Biopharm 17: 264-270.
- Hehre WJ, Yu J, Klunzinger PE, Lou L (2014) Spartan Pro. Wavefunction Inc. Irvine, Spartan 1.
- Exner O, Bohm S (2005) Protonated nitro group: structure, energy and conjugation. Org Biomolec Chem 3: 1838-1843.
- McOsker CC, Fitzpatrick PM (1994) Nitrofurantoin: Mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother 33: 23-30.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences