Anthocleista djalonensis a Potent Cholesterol Synthesis (Oxidosqualene Cyclase) Inhibitor - In Silico Docking and Molecular Dynamic Simulation Studies
Adebayo A Ogunboye1*, Mary T Olaleye1, Afolabi C Akinmoladun2, Olamide O Crown2 and Olanrewaju Sam Olayeriju3
1Department of Biochemistry, School of Life Sciences, Federal University of Technology, P. M. B. 704, Akure, Nigeria
2Department of Chemistry, Physics and Atmospheric Sciences. Jackson States University, Jackson, Mississippi, USA
3Department of Chemical Sciences (Biochemistry), School Sciences, Olusegun Agagu University of Technology, Okitipupa, Nigeria
- *Corresponding Author:
- Adebayo A Ogunboye
Department of Biochemistry,
School of Life Sciences,
Federal University of Technology,
P. M. B. 704,
Akure,
Nigeria,
Tel: 2349032632268;
E-mail: bayoogunboye2015@gmail.com
Received date: January 09, 2023, Manuscript No. IPJSVP-23-15621; Editor assigned date: January 12, 2023, PreQC No. IPJSVP-23-15621 (PQ); Reviewed date: January 27, 2023, QC No. IPJSVP-23-15621; Revised date: March 09, 2023, Manuscript No. IPJSVP-23-15621 (R); Published date March 16, 2023, DOI: 10.36648/2469-6692.9.1.002
Citation: Ogunboye AA, Olaleye MT, Akinmoladun AC, Crown OO, Olayeriju OS (2023) Anthocleista djalonensis a Potent Cholesterol Synthesis (Oxidosqualene Cyclase) Inhibitor–In Silico Docking and Molecular Dynamic Simulation Studies. In Silico In Vitro Pharmacol Vol:9 No:1.
Abstract
Plant sterols and stanols (phytosterols) are natural constituents of the plant cell membrane. From a chemical point of view, they are very similar to cholesterol, with minor differences in the relative positions of ethyl and methyl groups. Gas Chromatography-Mass Spectrophotometric (GC-MS) analysis, in silico docking and molecular dynamic simulation were carried out on Anthocleista djalonensis leaf. GC-MS analysis revealed the presence of twenty six phytochemicals consisting of phytosterols, sterol esters, unsaturated fatty acids, triterpenes and saturated fatty acids. Docking analysis showed that stigmasterol had the highest binding energy of 12.9 Kcal/mol among the identified phytochemicals and the simulated complexes revealed stability and ligand remaining inside the binding pocket. Molecular docking simulation revealed that stigmasterol effectively interacted with the targeted enzyme (oxidosqualene cyclase) in silico. Molecular interaction studies of the complexes indicated that the binding interactions between the compounds and our targeted proteins were found to be majorly hydrogen bonds and hydrophobic interactions. The result was analyzed using Root Mean Square Deviation (RMSD), Root Mean Square Fluctuation (RMSF), Radius of gyration (Rg) and Solvent Accessible Surface Area (SASA). These findings suggest that the leaf of Anthocleista djalonensis has phytochemicals which may be responsible for some of its reported pharmacological activities.
Keywords
Radius of gyration (Rg); Gas chromatography; Mass spectrophotometry; Oxidosqualene cyclase; Root Mean Square Fluctuation (RMSF)
Introduction
Plant sterols and stanols (phytosterols) are natural constituents of the plant cell membrane. From a chemical point of view, they are very similar to cholesterol, with minor differences in the relative positions of ethyl and methyl groups. Several mechanisms have been proposed to explain the cholesterol-lowering action of phytosterols. They may compete with dietary and biliary cholesterol for micellar solubilization in the intestinal lumen, impairing intestinal cholesterol absorption. Recent evidence indicates that phytosterols may also regulate other pathways. Impaired intestinal cholesterol absorption is usually associated with reduced cholesterol transport to the liver, which may reduce the incorporation of cholesterol into Very Low Density Lipoprotein (VLDL) particles, thereby lowering the rate of VLDL assembly and secretion. Impaired liver VLDL production may reduce the rate of LDL production [1].
It has recently been reported that accumulations of some phytosterols such as stigmasterol with an unsaturation within the side chain, disrupt cholesterol metabolism since they are endogenous regulators of the two major cholesterol regulatory pathways, LXR and SREBP. More than 200 different phytosterols have been identified stigmasterol and β-sitosterol is the most commonly occurring ones. A daily intake of 2.0-2.5 g phytosterols has been recommended for decreasing LDL cholesterol levels by up to 10% and lowering the risk of cardiovascular diseases [2].
A recent review also indicated that phytosterols are effective in lowering levels of plasma TG. It was reported that phytosterol treatment reduced serum and hepatic TG levels by modulating the expression of lipid regulatory genes and de novo lipogenesis. Phytosterols may also inhibit the absorption of fats by affecting bile acid homeostasis. Phytosterols exist universally in the roots, stems, foliage, fruits and seeds of various species of plants. Not only do they have the therapeutic effects of lowering serum cholesterol levels, but also have effects of anti-inflammatory, analgesia, preventing cardiovascular and cerebrovascular diseases, antioxidation, antineoplastic therapy, immunoregulation, skin protection and delaying senescence. Hence researches and exploitations to them are always hotspot in nature products researching field. In recent years, they have been widely used in medicine, food, cosmetics and other fields as drug intermediates and functional active factors [3].
The enzyme Oxidosqualene Cyclase (OSC) converts oxidosqualene to lanosterol, forming the steroid scaffold in a single reaction. In a search for drugs that reduce plasma cholesterol levels in humans, the enzyme was established as an interesting target besides squalene synthase, squalene epoxidase, and HMG-CoA reductase. In contrast to the reductase, the down regulation of OSC has only minor consequences for the synthesis of other required isoprenoids. Moreover, OSC inhibition avoids the accumulation of toxic sterol intermediates that would emerge if catalytic steps further down the pathway of cholesterol biosynthesis were inhibited. In addition to directly cyclizing 2, Monoepoxysqualene (MES) to lanosterol, this enzyme also catalyzes the conversion of 2, 3, 22,23 Diepoxysqualene (DES) into 24,25-epoxylanosterol. The latter is eventually transformed to 24,25-epoxycholesterol which is a known repressor of HMG-CoA reductase. Inhibition of OSC should then combine two synergistically acting mechanisms: Decrease of the amount of lanosterol formed (i.e., direct inhibition of cholesterol biosynthesis), and repression of HMGCoA reductase through the formation of oxysterols such as 24,25-epoxycholesterol (i.e., regulation of cholesterol biosynthesis). Oxidosqualene Cyclase Inhibitors (OSCI) arrest the downstream of 2,3-oxidosqualene which helps to stimulate epoxy sterols to repress HMG-CoA reductase expression. Partial inhibitors of oxidosqualene cyclase repress HMG-CoA reductase in HepG2 cells, possibly via the conversion of 2,3 oxidosqualene into oxysterols that inhibit HMG-CoA reductase. OSC represents a promising therapeutic target for hypocholesterolemic development since the cyclization is a post-squalene reaction in the sterol biosynthesis pathway. Thereby, inhibition of the human enzyme can avoid side effects observed for pre-squalene inhibition by the mostly used cholesterol-lowering drugs statins due to impairment of isoprenoids synthesis which are precursors of molecules involved in essential biological processes such as RNA transcription, ATP synthesis, protein N-glycosylation and prenylation. Besides, inhibition of OSC has been demonstrated to inhibit the cholesteryl ester deposition and foam cell formation without prejudicing triglycerides synthesis which could also contribute to prophylaxis of cardiovascular diseases through both mechanisms of action [4].
Anthocleista djalonensis A. Chev (Loganiaceae) is a mediumsized tree of the West tropical Africa, 30–45 feet high with blunt spines on the unbranch, pale grey trunk and wide spreading crown. Ethnomedicinally, the seeds, barks, leaves and roots are used as antipyretic, laxative, remedy for various stomach disorders, antihelmintic, antimycobacterial, antibacterial and wound healing. It is used as herbal remedy for epilepsy. The methanolic extract of A. djalonensisleaves showed a very potent DPPH and O2- anion radical scavenging activities. Also, the extract displayed significantly higher OH radical and nonenzymatic lipid peroxidation inhibitory potentials than that of standard antioxidants. It also inhibited the accumulation of nitrite in vitro. However, little is known about the antihyperlipidemic potential and its pharmacological mechanisms. Hence, this study seeks to establish the antihyperlipidemic potential of the leaf extract of Anthocleista djalonensis using the GC-MS analysis, in silico docking and molecular dynamic simulation studies [5].
Materials and Methods
Plant material
Leaves of Anthocleista djalonensis were collected from Yasere village in Okitipupa, (6°25’ and 6°25’ N latitude and 4°35’ and 4°50’ E longitude), Nigeria. The plant was authenticated by Omomoh Bernard of the department of crop, soil and pest management, the Federal university of technology Akure, Nigeria and a voucher specimen (FUTA-0150) was deposited in the herbarium [6].
Preparation of methanol extract of Anthocleista djalonensis Leaf (ADL)
The air-dried leaves were reduced to a coarse powder using a dry grinder. The powdered leaves were macerated in 80% methanol for 72 hours and filtered using Whatman filter paper no.1 to obtain the methanol extract of ADL. The filtered extract was concentrated in a rotary evaporator and further concentrated to dryness using a freeze dryer. The residue was kept for further studies. The yield of the extract was calculated [7].
Gas chromatography–tandem mass spectrometry of ADL
Gas chromatography–tandem mass spectrometry analysis of Anthocleista djalonensiswas done using a varian 3800 gas chromatograph equipped with a Agilent MS capillary column (30 m × 0.25 mm i.d.) connected to a Varian 4000 mass spectrometer operating in the EI mode (70 eV). The carrier gas was nitrogen with a constant flow rate of 1 ml min-1 with split ratio of 10:1. The oven temperature was operated according to the following oven temperature: 110°C held for 2 min, raising at the rate of 100°C min-1 up to 200°C with no held, raising at the rate of 5°C min-1 up to 200°C with 9 min held, injector temperature and volume 250°C and 1 μL, respectively. The total GC running time was about 36 min. The MS operating conditions were ionization voltage 70 eV, source temperature of 200°C, inlet line temperature of 200°C, mass scan (m/z) 45-450, solvent delay: 0-2 min, total MS running time 36 min. Compounds were identified by comparison of their mass spectra with those in the NIST 2005 library. All the samples and replicates were continuously injected as one batch in random order to discriminate technical from biological variations. Additionally, the prepared pooled samples were used as Quality Controls (QCs), which were injected at regular intervals throughout the analytical run to provide a set of data from which the repeatability can be assessed [8].
In silico study
Molecular docking: AutoDock Vina algorithm was used for computational analysis. The structures of the compounds were drawn and optimized into 3D using Marvin sketch suite and MMFF94 force field. The X-ray crystal structures of human oxidosqualene cyclase (PDB ID: 1W6K) with a resolution of resolution of 2.10 Å was retrieved from research collaboratory for structural bioinformatics (protein data bank). The water molecules were removed using pymol [9].
Molecular docking calculations using AutoDock vina 4.2 software: The protein structure and ligands were processed with AutoDock tools. Grids for docking evaluation with a spacing of 0.375 Å and 60 x 60 x 60 points, centered in the ligand binding pocket with X=29.64, Y=69.65, Z=8.94 coordinates for proteins downloaded from protein data bank. The procedure was carried out by considering the flexibility of the ligand such that all rotational bonds were set free and the estimated binding energies for the best pose were recorded. Docking runs were executed and the most favorable binding energies documented [10].
Docking pose analysis: The pose was analyzed using Pymol while the H-bond interactions and amino acid residues contributing to ligand binding were visualized using proteinligand profiler.
Molecular Dynamics (MD) simulation: The MD simulation was performed using GROMACS 4.6.5 MD package running on Ubuntu 18.04 (Hp-635 CPU@ 2.5 GHz Intel Core™ i5 RAM 6 GB) with GROMOS force field. SARS-CoV-2 PLpro was analyzed for MD simulation. The process started with initial cubic solvation with single point charge water model, followed by ionization and neutralization in Na and Cl ions (0.15 M). The solvated system was subjected to further energy minimization to remove stearic conflicts between protein and water molecules using the steepest descent integrator. The minimized model was subjected to position-restrained MD under NPT conditions by keeping the Number of particles (N), the system Pressure (P) and the Temperature (T) constant. This was executed for 100,000 steps for a total of 200 ps. The temperature of 300 k and a pressure of 1 atm were maintained. After equilibration of the system with constant temperature and pressure, the production MD run of 10,000,000 steps was performed for 30 ns to carry out the structural analysis on PLpro and its complex with the lead compound [11].
Results
GC-MS profile of Anthocleista djalonensis Leaf extract (ADL)
The GC-MS analysis of ADL revealed the presence of 26 major peaks indicating the presence of 26 phytochemical constituents. The 26 phytocompounds were characterized and identified. Some of the type compounds were:
• Phytosterols: Stigmasterol, campesterol.
• Terpenoids: Squalene, phytol.
• Fatty acid methyl esters: Hexadecanoic acid methyl ester.
• Fatty acids: Oleic acid, ricinoleic acid.
• Alkaloids: Theobromine.
And some other fatty acid esters compounds. Retention time, compound name, peak area, and percentage composition are stated in Table 1.
| S/N | Retention time | Compounds detected | Peak area (%) | % composition |
|---|---|---|---|---|
| 1 | 3.78 | Butanedioic acid, mono (2,2-dimethylhydrazide) | 0.58 | 2.92 |
| 2 | 9.46 | 3-O-Methyl-d-glucose | 0.61 | 38.05 |
| 3 | 13.21 | 2-Butenedioic acid, 2-methyl-, (Z)- | 1.46 | 2.99 |
| 4 | 15.84 | 3-Hydroxybenzaldehyde | 1.46 | 2.29 |
| 5 | 19.26 | Theobromine | 0.58 | 4.99 |
| 6 | 22.94 | Hexanoic acid, 3-hydroxy-, ethyl ester | 0.73 | 5.47 |
| 7 | 23.41 | caryophyllene | 0.76 | 2.22 |
| 8 | 25.68 | N-Hexadecanoic acid | 1.17 | 2.32 |
| 9 | 27 | Oleic acid | 0.73 | 2.08 |
| 10 | 28.63 | Phytol | 1.75 | 4.81 |
| 11 | 29.57 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 4.09 | 2.42 |
| 12 | 30.46 | Ricinoleic acid | 15.76 | 3.45 |
| 13 | 31.08 | Hexadecanoic acid, methyl ester | 3.8 | 4.88 |
| 14 | 33.41 | Octadecanoic acid, methyl ester | 2.34 | 0.14 |
| 15 | 34.26 | 9, 12-Octadecadienoic acid, methyl ester | 4.07 | 0.54 |
| 16 | 36.18 | 9-Octadecenoic acid (Z)-,methyl ester | 17.51 | 2.38 |
| 17 | 37.52 | 5-Ethyl-4-hydroxy-2-methyl-3 (2H)-furanone, acetate | 12.84 | 3.54 |
| 18 | 38 | Nonacos-1-ene | 5.84 | 1.74 |
| 19 | 40 | Squalene | 4.38 | 4.08 |
| 20 | 42.86 | Eicosanoic acid, methyl ester | 2.34 | 0.15 |
| 21 | 49.37 | Linoleic acid, trimethylsilyl ester | 4.07 | 1.42 |
| 22 | 52.85 | Beta-Tocopherol | 2.92 | 1.14 |
| 23 | 55.34 | Docosanoic acid, methyl ester | 2.34 | 1.1 |
| 24 | 57.2 | Campesterol | 2.31 | 0.05 |
| 25 | 60 | Stigmasterol | 3.07 | 0.13 |
| 26 | 64.63 | 9,19-cyclolanostan-3-ol acetate (3 beta) | 2.48 | 0.45 |
Table 1: Phytochemicals identified in the methanol leaf extracts of Anthocleista djalonensis by GC-MS analysis.
Molecular docking and simulation analyses
Molecular docking simulation revealed that stigmasterol effectively interacted with the targeted enzyme (oxidosqualene cyclase) in silico. Stigmasterol has the highest binding energy (-12.9 Kcal/mol) among the phytochemicals detected in Anthocleista djalonensis by GC-MS analysis (Table 2). Furthermore, the molecular interaction studies of the complexes indicated that the binding interactions between the compounds and our targeted proteins were found to be majorly hydrogen bonds and hydrophobic interactions. Also, Root Mean Square Deviation (RMSD) that is the measure of the average distance between the atoms (usually the backbone atoms) of superimposed proteins, Root Mean Square Flunctuation (RSMF) that is a measure of deviation between the position of particles and references position, radius of gyration that is the measure of compactness of the ligand and the enzyme and Solvent Accessible Surface Area (SASA) that is the surface area of a biomolecule that is accessible to a solvent were also measured [12].
| S/No | Compounds | Binding energy (Kcal/mol) |
|---|---|---|
| 1 | 2-Butenedioic acid, 2-methyl-, (Z)- | -5.2 |
| 2 | 3-Hydroxybenzaldehyde | -6.1 |
| 3 | 3-O-Methyl-d-glucose | -5.4 |
| 4 | Hexanoic acid, 3-hydroxy-, ethyl ester | -5.4 |
| 5 | Caryophyllene | -8.5 |
| 6 | N-Hexadecanoic acid | -7.2 |
| 7 | Oleic acid | -8 |
| 8 | Phytol | -8.3 |
| 9 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | -8.5 |
| 10 | Ricinoleic acid | -8.1 |
| 11 | Hexadecanoic acid, methyl ester | -7.2 |
| 12 | Octadecanoic acid, methyl ester | -7.5 |
| 13 | 9-Octadecenoic acid (Z)-,methyl ester | -7.6 |
| 14 | 5-Ethyl-4-hydroxy-2-methyl-3(2H)-furanone, acetate | -6.6 |
| 15 | Nonacos-1-ene | -8.2 |
| 16 | Squalene | -10.6 |
| 17 | Eicosanoic acid, methyl ester | -7.4 |
| 18 | Linoleic acid, trimethylsilyl ester | -7.6 |
| 19 | Beta-Tocopherol | -10.3 |
| 20 | Docosanoic acid, methyl ester | -7.6 |
| 21 | Campesterol | -11.9 |
| 22 | Stigmasterol | -12.9 |
| 23 | 9,19-cyclolanostan-3-ol acetate(3 beta) | -8.5 |
| 24 | Theobromine | -6.8 |
| 25 | Hexadecanoic acid, methyl ester | -7.2 |
| 26 | 9, 12-Octadecadienoic acid, methyl ester | -8 |
Table 2: Binding energy of Anthocleista djalonensis derived compounds using GC-MS analysis with human oxidosqualene cyclase.
Interaction of stigmasterol with human oxidosqualene cyclase
Figure 1 shows the 2D structure of stigmasterol and human oxidosqualene cyclase.
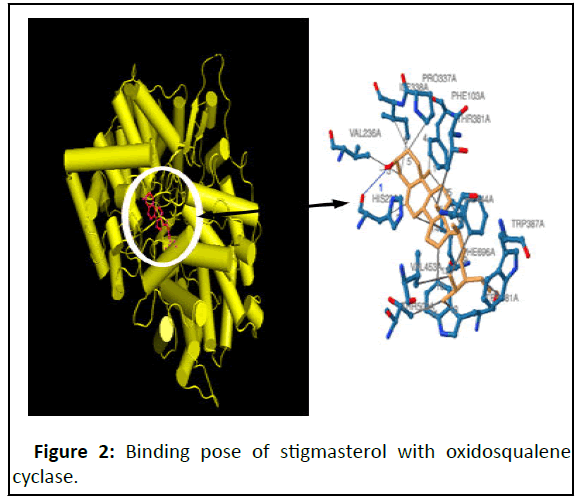
Figure 2 shows the binding pose of ligands (stigmasterol) with oxidosqualene cyclase, a key enzyme in the cholesterol biosynthesis pathway that catalyzes the formation of lanosterol, which is then converted through many steps into cholesterol.
Figure 3 and Table 3 showed the molecular simulation results for the interaction between stigmasterol and oxidosqualene cyclase. Figure 4 illustrates the overall pattern of the interaction. The interaction pocket where stigmasterol bound to oxidosqualene cyclase contained the following amino acids: Phe103A, His232A, Val236A, Pro337A, Ile338A, Ile338A, Thr381A, Trp387A, Phe444A, Val453A, Thr502A, Trp581A, Trp581A, Phe696A, Phe696A, Phe696A, Phe696A, Phe696A. Table 4 showed the phyto constituents of ADL and their respective binding energy. Table 3 showed the hydrophobic interaction of stigmasterol (amino acids, distance ligand atom and protein atom).
| Index | Residue | AA | Distance | Ligand atom | Protein atom |
|---|---|---|---|---|---|
| 1 | 103A | PHE | 3.85 | 5837 | 766 |
| 2 | 232A | HIS | 3.75 | 5826 | 1788 |
| 3 | 236A | VAL | 3.87 | 5822 | 1826 |
| 4 | 337A | PRO | 3.86 | 5820 | 2662 |
| 5 | 338A | ILE | 3.63 | 5822 | 2671 |
| 6 | 338A | ILE | 3.6 | 5820 | 2668 |
| 7 | 381A | THR | 3.53 | 5836 | 3015 |
| 8 | 387A | TRP | 3.31 | 5843 | 3062 |
| 9 | 444A | PHE | 3.79 | 5847 | 3526 |
| 10 | 453A | VAL | 3.75 | 5847 | 3596 |
| 11 | 502A | THR | 3.5 | 5846 | 3968 |
| 12 | 581A | TRP | 3.05 | 5834 | 4621 |
| 13 | 581A | TRP | 3.75 | 5846 | 4616 |
| 14 | 696A | PHE | 3.96 | 5827 | 5520 |
| 15 | 696A | PHE | 3.63 | 5837 | 5524 |
| 16 | 696A | PHE | 3.77 | 5825 | 5522 |
| 17 | 696A | PHE | 3.9 | 3831 | 5519 |
| 18 | 696A | PHE | 3.83 | 5829 | 5521 |
Table 3: Hydrophobic interaction and hydrogen bond of stigmasterol-oxidosqualene cyclase complex showing amino acid residue, distances and ligand atoms.
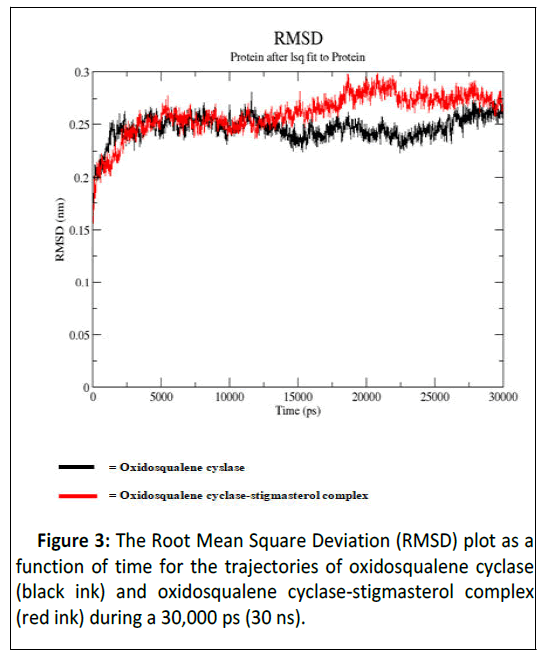
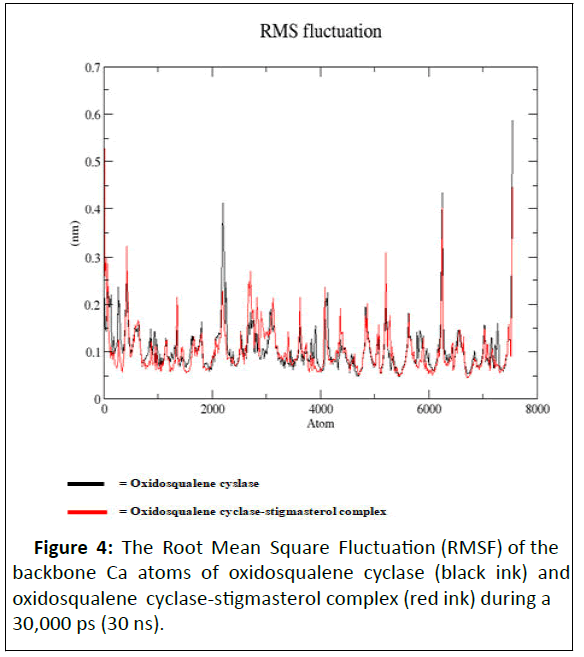
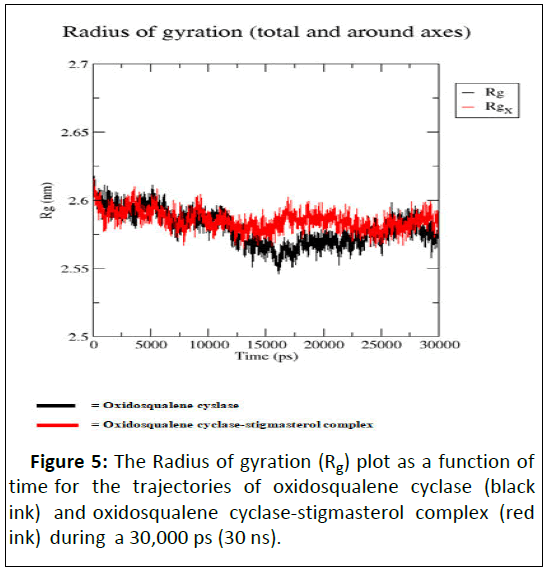
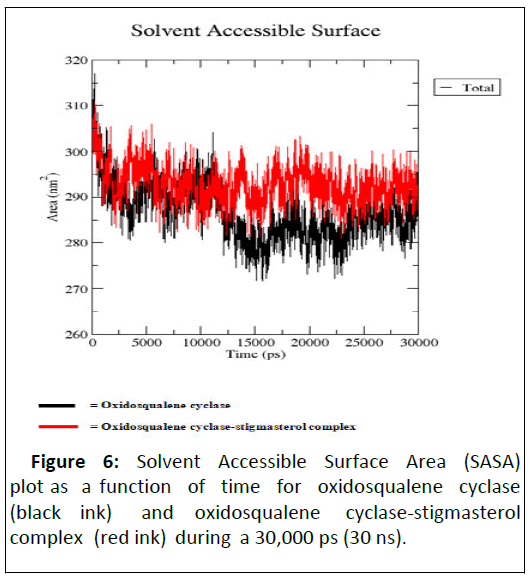
As illustrated by docking studies, stigmasterol showed strongest affinity with human oxidosqualene cyclase pockets along with highest binding scores. To investigate the validity of the docking data and results, the docked complexes of stigmasterol with oxidosqualene cyclase were simulated for 30 ns and 30,000 ps (Figures 3-6). Figure 3 is the Root Mean Square Deviation (RSMD) showing the average distance between stigmasterol and oxidosqualene cylase in forming the stigmasterol oxidosqualene cyclase complex. Figure 4 is the Root Mean Square Flunctuation (RMSF) showing a measure of deviation between the position of particles and references position. Figure 5 is the Radius of gyration (Rg) showing the measure of compactness of stigmasterol and oxidosqualene cyclase to form the stigmasterol-oxidosqualene cyclase complex. Figure 6 showed the Solvent Accessible Surface Area (SASA) that is the surface area of a biomolecule that is accessible to a solvent of stigmasterol with oxidosqualene cyclase (Table 4).
| Index | Residue | AA | Distance H-A | Distance D-A | Donor angle | Protein donor? | Side chain | Donor atom | Acceptor atom |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 232A | HIS | 2.92 | 3.71 | 137.78 | X | X | 5848 | 1787 |
Table 4: Hydrogen bonds.
Discussion
Gas Chromatography (GC) and Mass Spectrometry (MS), is used to analyze complex organic and biochemical mixtures. The GC-MS analysis of methanol extract of ADL revealed the presence of butanedioic acid, mono (2, 2-dimethylhydrazide, theobromine, 3-O-methyl-d-glucose, 2-butenedioic acid 2- methyl-(Z)-, 3-hydroxybenzaldehyde, hexanoic acid, 3-hydroxy-, ethyl ester, caryophyllene, N-hexadecanoic acid, oleic acid, phytol, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, ricinoleic acid, hexadecanoic acid methyl ester, octadecanoic acid methyl ester, 9, 12-octadecadienoic acid methyl ester, 9-octadecenoic acid (Z)- methyl ester, 5-ethyl-4-hydroxy-2-methyl-3(2H)-furanone acetate, onacos-1-ene, squalene, eicosanoic acid methyl ester, linoleic acid trimethylsilyl ester, beta-tocopherol, docosanoic acid methyl ester, campesterol, stigmasterol, and 9,19- cyclolanostan-3-ol acetate (3 β). Some of the classes of these compounds include sterols (e.g. stigmasterol, campesterol), unsaturated fatty acids (e.g. oleic acid, linoleic acid), terpenes (e.g. squalene, phytol) and fatty acids esters (e.g. 9,12,- octadecadienoic acid methyl ester, octadecanoic acid methyl ester, hexadecanoic acid methyl ester), fatty acids (e.g. Nhexadecanoic acid).
Plant sterols are of good therapeutic options for the management of hypercholesterolemia while triterpenes possess cytotoxic activity. Sterol esters are a heterogenous group of phytosterol esters with a saturated ring structure which are known to reduce low density lipoprotein cholesterol. The ability of phytosterols (plant steroids) like stigmasterol, campesterol and β-sitosterol to reduce cholesterol levels was first demonstrated in humans in 1953. Today, it is well established that high intakes of plant sterols or stanols can lower serum total and LDL cholesterol concentrations in humans. In the intestinal lumen, phytosterols displace cholesterol from mixed micelles and inhibit cholesterol absorption.
Molecular docking studies are used to determine the interaction of two molecules and to find the best orientation of ligand, which would form a complex with overall minimum energy. When a ligand molecule is docked at the active site of macromolecular targets, conformational changes may occur in some of the amino acid residues at the active site. In order to find the best fit at the active site, flexibility has to be provided not only for the ligand but also for the active site residues.
Targeting the sterol biosynthesis pathway has been explored for the development of new bioactive compounds. Among the enzymes of this pathway, Oxidosqualene Cyclase (OSC) which catalyzes lanosterol cyclization from 2,3-oxidosqualene has emerged as an attractive target. The enzyme’s genetic expression is regulated by Sterol Regulatory Element Binding Protein (SREBP-2), a molecule which also regulates the expression of other enzymes in the cholesterol biosynthesis pathway. This is largely because blocking the cholesterol biosynthesis pathway before squalene has been found to disrupt the synthesis of isoprenoids, which are used for the biosynthesis of key molecules in RNA transcription, ATP synthesis, and other essential cell activities.
Oxidosqualene cyclase, which is downstream of squalene in the pathway, is an attractive target for inhibition. Many inhibitors have been proposed, among them steroid analogs, phenol based compounds, benzamide and carboxamide derivatives, and nitrogen-containing heterocyclic compounds. The most effective inhibitors have a hydrogen-bond acceptor at a specific distance away from a hydrophobic region. The molecular docking showed that phytosterols such as stigmasterol detected in ADL are potential oxidosqualene cyclase inhibitors. This deduction was determined by the binding affinity, amino acid residues, and hydrophobic interactions between each ligand and receptor. Among the phytochemical constituents of ADL revealed by GC-MS analysis, stigmasterol has the highest binding energy on human oxidosqualene cyclase. Stigmasterol interacted with Phe103A, His232A, Val236A, Pro337A, Ile338A, Ile338A, Thr381A, Trp387A, Phe444A, Val453A, Thr502A, Trp581A, Trp581A, Phe696A, Phe696A, Phe696A, Phe696A, Phe696A residues at the binding cavity with binding energy of -12.9 Kcal/mol which indicates good binding affinity with the active site residues in human oxidosqualene cyclase and the formation of hydrogen bonds with amino acid residues as observed in this research. The amino acid residues indicated that the stigmasterol in ADL was able to bond to the active site of the receptor. This interaction proved that ligands play an important role in inhibiting protein function.
Hydrophobic interactions formed between ligand-receptors serve to stabilize ligands in conformation to protein structure and increase ligand-receptor binding affinity and increase biological activity from ligands. The docking results of stigmasterol with oxidosqualene cyclase showed that it is a potent inhibitor of oxidosqualene cyclase and can prevent hyperlipidemia caused by cholesterol biosynthesis through its pleiotropic effects i.e. preventing cholesterol biosynthesis by inhibiting other downstream enzymes and not only HMGCoA reductase, the rate limiting enzyme.
Conclusion
The Root Mean Square Deviation (RMSD) between oxidosqualene and oxidosqualene cyclase was close at 10 ns but increased between 15 ns and 25 ns. However at 30 ns, this deviation became close. Stigmasterol-oxidosqualene complex retained less deviation at 10 ns resulting in a back bone of 2 to 2.5 nm (A). This shows that the complex maintain it stability at 10 ns and ability to prevent the activity of oxidosqualene cyclase cholesterol biosynthesis. Stigmasterol maintained less fluctuation compared with oxido squalene cyclase revealing the ability to form a complex as degree of flexibility proved the stability of stigmasterol oxidosqualene cyclase complex and the ability of stigmasterol to prevent the generation of cholesterol. The Radius of gyration (Rg) is the mass-weighted root mean square distance of group of atoms from their common centre of mass. Hence it provides an observation into global dimension of protein. In the case of stigmasterol, no major fluctuation is observed in stigmasterol oxidosqualene cyclase complex and this makes it to become stable. Solvent Accessible Surface Area (SASA) of the stigmasterol oxidosqualene cyclase complex was also studied. SASA is the locus of the center of the solvent molecule as it rolls over the surface of the protein. The result showed that stigmasterol was able to access the surface closely while forming oxidosqualene stigmasterol complex. Oxidosqualene cyclase is an enzyme which plays a role in the biosynthesis of lipid and its inhibition could reduce the production of cholesterol. Therefore, interaction of stigmasterol (one of the bioactive compounds in ADL) with oxidosqualene cyclase as observed in this study could be one of the therapeutic targets for hyperlipidemia.
References
- Bajda M, Boryczka S, Malawsk B (2007) Investigation of lipophilicity of anticancer-active thioquinoline derivatives. Biomed Chromatogr 21:123-131
[Crossref] [Google Scholar] [PubMed]
- Bruno-Blanch L, Galvez J, Garcia-Domenac R (2003) Topological virtual screening: A way to find new anticonvulsant drugs from chemical diversity. Bioorg Med Chem Lett 13: 2749-2754
[Crossref] [Google Scholar] [PubMed]
- El-Baih FEM, Al-Rasheed HH, Hassan MA (2006) Microwave assisted synthesis of substitutefuran-2-carboxaldehydes and their reactions. J Saudi Chem Soc 9:575
- Estrada E, Pena, A (2000) In silico studies for the rational discovery of anticonvulsant compounds. Bioorg Med Chem 8:2755-2770
[Crossref] [Google Scholar] [PubMed]
- Mahapatra A, Prasad T, Sharma T (2021) Pyrimidine: A review on anticancer activity with key emphasis on SAR. Future J Pharm Sci 7:1-38
- Gore RP, Rajput AP (2013) A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Inven Today 5:148-152
- Martins MAP, Cunico W, Pereira CMP, Flores AFC, Bonacorso HG, et al. (2004) 4-alkoxy-1,1,1-trichloro-3-alken-2-ones: Preparation and applications in heterocyclic synthesis. Curr Org Synth 1:391-403
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, et al. (2019) Cancer treatment and survivorship statistics. CA Cancer J Clin 69:363–385
[Crossref] [Google Scholar] [PubMed]
- MohanaRoopan S, Sompalle R (2016) Synthetic chemistry of pyrimidines and fused pyrimidines: A review. Synth Commun 46:645–672
- Prachayasittikul S, Pingaew R, Worachartcheewan A, Sinthupoom N, Prachayasittikul V, et al. (2017) Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev Med Chem 17:869–890
[Crossref] [Google Scholar] [PubMed]
- Dinakaran VS, Bomma B, Srinivasan KK (2012) Fused pyrimidines: The heterocycle of diverse biological and pharmacological significance. Der Pharma Chem 4:255-265
- Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55-63
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences