Effects of Calcineurin Inhibitors on Rat Thoracic and Abdominal Aortae Contractibility and Vasodilatation
Jadhav A, Gopalakrishnan V, Shoker A
DOI10.21767/2469-6692.10002
Jadhav A1*, Gopalakrishnan V1 and Shoker A2
1Department of Pharmacology, College of Medicine, University of Saskatchewan, Saskatchewan, Canada
2Department of Medicine, University of Saskatchewan in Saskatoon, Saskatchewan, Canada
- *Corresponding Author:
- Ashok Jadhav
Department of Pharmacology, College of Medicine
University of Saskatchewan, Saskatchewan, Canada
Tel: (306)-966-6294
Fax: (306)-966-4298
E-mail: ashok.jadhav@usask.ca
Abstract
Context: Calcineurin inhibitors (CNIs) therapy such as cyclosporine (CsA) and tacrolimus (Tac) are associated with hypertension.
Objective: Compare the differential effect of these two agents on thoracic and abdominal aortae.
Methods: Thirteen weeks old male Sprague-Dawley rats were treated with CsA (15 mg/kg), Tac (0.15 mg/kg) and vehicle for 15 days. At the end of study, rats were anesthetised, and blood pressure (BP) and heart rate (HR) were measured. Both thoracic (TA) and abdominal (AA) aortae were assessed for the contractibility responses with α1 agonist phenylephrine (PE) against terazosin, a α1 receptor blocker. In addition, vascular relaxation was assessed using endothelium-dependent, Acetylcholine-induced and
endothelium-independent sodium nitroprusside-induced pathways in PEconstricted TA and AA.
Results: Elevated arterial BP was noted in Tac group, however, HR did not showed any changes in Tac and CsA groups as compared to controls. PEinduced contractibility of TA but not AA was increased significantly and equally by CsA (EC50, 55 ± 7.06 nM) and Tac (EC50, 50 ± 6.6 nM) treatment as
compared to control (104 ± 14 nM). ACh-induced endothelium-dependent relaxation was attenuated by both CsA (TA: Imax 66 ± 8.79 %, AA: Imax 74 ± 11.38 %) and Tac (TA: Imax 39 ± 9.55 %, AA: Imax 70 ± 20.06 %), however its intensity was higher in TA by Tac group (controls: 97-98 ± 2.6-2 %). Similarly, SNP-induced endothelium-independent vascular relaxation was also affected by CsA and Tac treatment, TA (IC50 control, 47 ± 11.2 nM, vs. CsA, 214 ± 27.9 nM and Tac, 191 ± 30.8 nM, p< 0.05) and AA (IC50 control, 164 ± 45.1 nM, vs. CsA, 424 ± 53.4 nM and Tac, 520 ± 104.1 nM, p< 0.05).
Conclusions: Our functional data confirms that CNIs have differential effects on rat TA and AA via either alternation of vascular relaxations or contractions.
Keywords
Cyclosporine; Tacrolimus; Calcineurin; Hypertension; Acetylcholine; Sodium nitroprusside; Aorta
Abbreviations
Acetylcholine, ACh; Phenylephrine,PE; Calcineurin Inhibitors,CNIs; Sodium Nitroprusside, SNP; Dose Response Curve, DRC; Cyclosporine, CsA; Tacrolimus, Tac; Heart rate, (HR); Thoracic Aorta, TA; Abdominal Aorta, AA; EC50, Half Maximal Effective Concentration; IC50, Half Maximal Inhibitory Concentration; Emax, Maximum Possible Effect for the Agonist; Imax, Maximum Inhibitory Effect for the Agonist
Introduction
Calcineurin inhibitors (CNIs) such as cyclosporine (CsA) and tacrolimus (Tac) have been used clinically as immunosuppressive regimen for the solid organ transplantation [1]. Their efficacy against the transplant rejection comes with the cost of side effect such as hypertension and vascular injury [2,3]. The incidence of hypertension in liver or heart recipients are 80-90 % when treated with CsA [4], whereas 50-70 % with Tac treatment [5]. Studies have also shown that the hypertension caused due to CNIs are multifactorial which also includes direct effect on the vasculature [6-8]. It is either by an enhanced activity of vasoconstrictive agent such as endothelin-1 [9] or via inhibiting nitric oxide [NO] synthesis [10]. The generation of NO is constitutive in the vascular endothelium, the Ca2+-dependent inducible NO synthase (iNOS) from the endothelium plays an important role in the maintenance of vascular homeostasis. Shear stress and receptor stimulation to endothelial NO synthase (eNOS) do release a small amount of NO, which also contributes to maintain vascular tone. In fact the inhibition of NOS leads to generalized vasoconstriction and significantly increases blood pressure [BP] [10,11]. The vascular injury caused due to CsA treatment were showed in different in vivo model of renal function [12-15], isolated perfused kidney [12], and hydronephrotic kidney [13]. The in vitro chronic effect of CsA on isolated vascular rings were also demonstrated using isolated thoracic aortae [TA] [14,15] and or with renal arteries [16], interlobar arteries [17], mesenteric resistance arteries [18], and femoral arteries [19]. Although, some of these studies have showed that the CsA has vascular toxicity effects while others showed no vascular alterations. One of the important vascular toxicity caused due to CsA is the impairment of endotheliumdependent acetylcholine (ACh)-induced vascular relaxation, which is also depends on integrity of endothelium and NO. CsAinduced impaired relaxation to ACh was found in TA, which was very sensitive to vasculotoxicity but its effect was not found in abdominal aortae (AA) and other vessels [20]. In contrast to NO-induced vascular actions, vascular noradrenalin receptors also plays important role in the regulation of vascular tone. CsA enhances vascular contraction by stimulating noradrenaline in rat aorta [19]. It also decreases vascular sensitivity to noradrenaline without changing maximal contractility [20]. Rats treated with CsA 30 mg/kg/d for 3 weeks caused decrease in the aortic rings response to phenylephrine (PE) (Figure 1) [14]. Conversely, increase in PE-induced contractions was noted in rabbit renal artery at the concentration of 1-10 μM of CsA without changing pD2 (potency of agonist) values [21]. In addition, an increase in vascular sensitivity to adrenergic agonists in the presence of CsA was also showed by Lamb and Webb, 1987 [22]. Surprisingly, there are no functional studies available to characterize α1 adrenoreceptor mediated vascular contractibility using two CNIs such as CsA and Tac on TA and AA. After careful reviewing of all these studies, we found that there are discrepancies which depends on difference in experimental conditions such as dose, duration of the treatment, or route of administration etc. [20]. Very few studies or none are available with respect to other CNIs such as Tac and its comparism with CsA on different portion of aortae such as TA and AA. Our previous studies using these two CNIs via in vitro study showed that CsA exerted profound inhibitory effect on endothelium-dependent vasodilatation in both TA and AA as compared to Tac [23]. To expand our research, we have decided to inject CsA and Tac subcutaneously in the in vivo rat model. Isolate both thoracic and abdominal aortae for the assessment of CNIs-induced inhibition of vasodilatation as well as functional alterations of vascular noradrenalin receptor activity. Therefore, the aim of the current study was to determine the effect of CNIs on different portion of Sprague Dawley (SD) rats’ aortic vessel (TA and AA) contractibility and relaxation. The functional contractibility was assessed using terazosin, an adrenergic receptor blocker, whereas relaxation effects were characterized using endothelium-dependent, ACh-induced and endothelium-independent, sodium nitroprusside (SNP)-induced vascular smooth muscle relaxation in both TA and AA.
Figure 1: The panel compares blood pressure parameter of different groups treated with vehicle (castor oil, s/c, n=5), cyclosporine (CsA-15 mg/kg, s/c, n=6) and tacrolimus (Tac-0.15 mg/kg, s/c, n=5) for 15 days. At the end of study, rats were anesthetized using inhalant anesthesia (isoflurane) and arterial blood pressure (ABP) was measured using femoral artery canulation. Heart rate (HR), Systolic blood pressure (SBP) and Diastolic blood pressure (DBP) were calculated using cyclic measurement on Chart5 software. ABP and DBP of TAC treated group were significantly different as compared to control-vehicle group. *p<0.05 vs. control-vehicle. Each data point is mean ± SEM value obtained from n=5-6 rats.
Materials and Methods
Animals
The current experiments were conducted in 13-week-old male Sprague-Dawley rats (300 to 350g) obtained from Charles River Laboratories (St. Constant, Quebec, Canada). Given experimental protocol was approved by Animal care committees at the University of Saskatchewan as the guideline stipulated by the Canadian Council on Animal Care. In present study, all the rats were handled and injected by same researcher, as well as in vitro experiment, in the same laboratory, using the same organ bath apparatus.
Materials
All the analytical grade salts used in the preparation of Krebs’ buffer, terazosin hydrochloride, phenylephrine hydrochloride (PE), sodium nitroprusside (SNP) and acetylcholine chloride (ACh) were obtained from Sigma-Aldrich Canada Ltd. (Oakville, Onatrio, Canada). tacrolimus (Prograf, i.v.) purchased from Astellas Pharma Canada, Inc (Markham, Ontario, Canada) and cyclosporine (Novartis, Sandimmune* i.v.) obtained from Novartis Pharmaceuticals, Canada Inc. (Dorval, Quebec, Canada), Other materials such as isoflurane USP inhalation anesthetic bought from Abbott Laboratories, Limited, (Montreal Canada,) and heparin sodium injection USP, from Sandoz, Canada Inc. (Qc, Canada). Polyethylene tubing (PE50) purchased from Becton Dickinson and Company, Sparks, MD and Silicon tubing (PE50) got from VWR international, Canada. PRISM software (Graph Pad Inc., La Jolla, CA) was used for the statistical analysis of data.
In-vivo study
In the in vivo study, 13 weeks male SD rats were divided into three groups, control-vehicle/castor oil, n=5 rats; CsA,15 mg/kg B.wt., n=6 rats; and Tac, 0.15 mg/kg B.wt., n=5 rats and were treated for 15 days subcutaneously (s/c). At the end of study, rats were anesthetised using inhalant anesthesia, and blood pressure and heart rate were measured as per method described below. Later rats were overdosed with isoflurane, and both TA and AA were isolated carefully without damaging vessels for in vitro organ bath study (Figure 1). A large part of our study has been conceived to evaluate the effect of CNIs on aortae reactivity. We have used anesthesia and surgery to isolate both TA and AA, which may influence the vascular tone of both aortic segments before their isolation. Therefore, we would like to acknowledge the limitation of our study.
Measurement of blood pressure (BP) and heart rate (HR)
At the end of treatment period of 15 days rats were anaesthetized with isoflurane gas (5% induction and 2 % maintenance mixed with Oxygen, n=16 rats) and used for measurement of blood pressure parameters. The anaesthetized rats were placed on a heating pad to maintain the temperature at 37oC (measured by a rectal probe) and were allowed to stabilize. Further, the right femoral artery and vein were cannulated with polythene cannulas. The femoral artery catheter (polyethylene tubing, i.d. 0.58 mm and o.d. 0.965 mm) was filled with heparinized saline (50 U/ml). It was connected to a pressure transducer to record the arterial blood pressure (mmHg) and HR (beat per min, BPM) using the Powerlab data acquisition system (AD Instruments Pvt. Ltd., Sydney, Australia). The femoral vein catheter (silicon tubing 0.20 ID X 0.037 OD X 0.008 Wall) was filled with heparinized saline and infused to balance the fluid losses during the catheterization. The experiment were continued for 10-15 minutes to obtain stable blood pressure (arterial BP) and was recorded on chart5 software. Heart rate, systolic and diastolic blood pressures were calculated from direct arterial BP channel using cyclic measurement parameter on chart5 program. The detailed methodology for the measurement of BP and HR were described earlier; however incorporation of isoflurane anesthetics agent were done recently in our laboratory [24,25].
Isolation of rat thoracic and abdominal aortae and organ bath technique
In current experiment rats were treated with vehicle, CsA and Tac for 15 days by s/c injections. After BP measurement rats were euthanized with overdose of isoflurane anesthetic agent. Both TA and AA aorta (separated at the diaphragm level) were isolated and placed into to petri plate containing buffer along with continue carbogen flow. Aortic rings cleaning, mounting, preload tension and endothelium viability of ring was done as method described below. Importantly, hooks were inserted in the aortic rings without damaging the endothelium. Aortic rings were maintained at the basal preload tension of 2 g in organ baths containing 10 ml Krebs buffer (in mM: 120, NaCl; 4.8, KCl; 1.2, MgCl2; 1.8, CaCl2; 1.2, KH2PO4; 25, NaHCO3; 11, glucose; pH 7.4 gassed with 95% O2, 5% CO2 at 37°C) as described previously (24, 25, 26). Later all rings were first contracted with a submaximal concentration (~EC80 level) of α1 selective agonist, PE (1 μM). Once steady state response achieved by aortic rings, the tissues were washed 3 times in normal krebs’ buffer and maintained for 1 h. Same concentration of PE was added in organ bath to ascertain that the sustained tonic response to PE is reproduced and a fixed concentration of ACh (10 μM) was added to assess the extent of vasodilatation in PE-constricted rings. If vasodilatation to ACh was >90%, it was considered as an endothelium-intact preparation [24-26]. After initial confirmation of both contractile and relaxation response further studies were performed as per methods described below.
Aortic ring contractile function
Some of TA and AA rings were exposed to PE- concentration response curve in the presence and absence of Terazosin (selective α1 receptor blocker). The EC50 (EC50, half maximal effective concentration) values were obtained were used to calculate concentration ratio (EC50 of Terazosin treatment/ EC50 of Control (Before treatment). In the experiment these aortic rings were exposed to PE cumulatively increasing concentrations (1 nM-100 μM) which were added in such a way that the next concentration was added only after the response to the previous concentration had plateaued. The data collected were considered as control (before treatment) and compared with after treatment of terazosin 10 nM or 30 nM drugs (selective α1 receptor inhibitor). Tissues were washed 3 times and allowed to recover by repeated washing for a minimum of 1 h. The tissues were incubated with either terazosin (10 nM) or (30 nM) concentration in 10 ml organ bath containing krebs’ buffer for 20 minutes. Later tissues were constricted with PE by adding cumulatively increasing concentrations (1 nM-100 μM) until it reaches maximal contraction which was achieved during control concentration response curve of PE. Thus, the changes in isometric tension evoked by PE (PE-induced concentration response curve plotted) in presence or absence of terazosin (10 and 30 nM) and were determined in the vessels of the same rat (n=5-6 rats). The tension responses were recorded in gram on a chart 5 program (Chart V5.0.1) using a Powerlab/8SP data acquisition system (AD Instruments Pvt. Ltd., Sydney, Australia). The maximum gram tension of control curve (Before terazosin treatment) were considered as 100 (percentage, %) and rest of lower values were normalized/converted into % of maximum value. Similarly, gram tension data from terazosin, 10 and 30 nMincubated, PE-concentration response curve were converted into %. Emax (maximum possible effect for the agonist) were calculated by taking average of maximum contractile response (%) to PE in each treatment group. Whereas, the EC50 values were obtained using PRISM software and used to calculate concentration ratio [EC50 of Terazosin treatment/ EC50 of Control (Before treatment)] [24-26].
Aortic ring relaxation function
Remaining TA and AA rings were used to assess vascular relaxation response of ACh (endothelium-dependent) and SNP (endothelium-independent) after a steady tonic response was reached following the addition of PE (1 μM), cumulatively increasing concentrations of either ACh (1 nM-100 μM), SNP (1 nM-100 μM) were added in such a way that the next concentration was added only after the response to the previous concentration had plateaued. The tension responses were recorded in gram on a chart programme (Chart V4.0.1) using a Powerlab/8SP data acquisition system (AD Instruments Pvt. Ltd., Sydney, Australia). The maximum gram tension achieved by PE were considered as 100 (%) and decrease in tension caused due to ACh and SNP were normalized/converted into % and used as % inhibition. The inhibitory concentration maximum (Imax) values were calculated by taking average of maximum reduction in tension caused due to ACh or SNP in each treatment group. Further, all % normalized data of each group were inserted in the PRISM statistical software to calculate inhibitory concentration of 50 % (IC50) and to plot concentration response curve graph. All these data collected were considered as the vascular relaxation actions of both CsA and Tac after the 15 days treatment s/c ly and compared with control-vehicle data [24-26].
Statistic
For in vivo study, average of arterial, systolic and diastolic BP, and HR were determined per group and the pooled values are expressed as mean ± SEM (n=5-6 rats). The data were analyzed for statistical significance using one-way ANOVA as the same variable (ABP or HR change) was compared between control and treatments followed by Tukey post hoc test and the differences between means was considered significant when the P value was < 0.05. These results were presented as column graphs. The closest P value obtained was given in the results section. For in vitro study, experiments were performed each day using both TA and AA aortic vessels after they were isolated from one rat and tested for contractile tension responses in PE-constricted concentration response curve. The contractile responses observed by PE were normalized as % change in tonic response to the basal reading evoked by cumulative concentration(s) of PE. The tonic response was reached closer to 100% and Emax value was generated by taking average % maximum contraction caused by PE agonist. The EC50 values were generated from each concentration response curve using Prism software.
Note: How to use Prism software for the analysis of curves. To get EC50 value of concentration response curve we should open Prism software file then select XY table and graph. The concentration of agonist should be inserted in the X column whereas responses (% contraction) in the Y column. Then click on icon (Analyze data) we will get a pop-up window, then select a parameter-transform the data (X=LogX). Later click on “OK” button. It will convert X value (concentration of agonist) into logarithmic format. Again, go back to icon (Analyze data) and select XY analyses (Nonlinear regression) curve fit. Then select Dose-response stimulation [log (agonist) vs. response] and click “OK). We will get 3 different files 1. Data tables, 2. Results and 3. Graphs. In the result section we will find the EC50 values for each group. Vascular relaxation study were performed each day by using both endothelium-dependant and endothelium-independent aortic vessels after they were isolated from one rat and tested for inhibition of tension responses in PEconstricted states. The vasodilator responses observed by ACh and SNP were normalized as % inhibition of steady state tonic response (100%) evoked by fixed concentration(s) of PE. The Imax, values were calculated by taking average of maximum % inhibition per group. Whereas IC50 values were generated by using Prism software as per procedure described above except we have used Dose-response inhibition [log (inhibitor) vs. response] curve instead of Dose-response stimulation [log (agonist) vs. response] curve to calculate IC50 values. Thus, the final mean ± SEM values shown in the results section represent the data gathered from 5-6 rats (5-6 tissues of thoracic aortae and abdominal aortae per rat). The differences in mean ± SEM values between different conditions of treatment were analysed using one-way ANOVA, followed by Tukey post hoc test and the data were considered significant when the P value was < 0.05. However, for assigning the level of significance the closest P value reached was provided in the results section.
Results
Drug concentration and blood pressure parameters
After 15 days of CNIs treatment (control-vehicle, CsA-15 mg/ kg and Tac-0.15 mg/kg), SD rats were anesthetised and BP parameters were monitored for 10 minutes. We found that, the direct arterial BP of control-vehicle group was 97.4 ± 2.20 mmHg. The CsA treated group showed no difference in ABP, 104 ± 3.35 mmHg, however Tac treated group (110 ± 3.3 mmHg, p<0.05, n=5-6) was significantly higher than the control group (Figure 1). This changes in arterial BP might be also be contributed due to changes in diastolic BP in the Tac group (control, 81 ± 2.65, CsA, 89 ± 3.52 and Tac, 94 ± 3.28 mmHg, p<0.05, n=5-6). However, there was no difference in systolic BP (control-vehicle, 125 ± 3.48, CsA, 127 ± 3.10 and Tac 132 ± 4.37 mmHg, n=5-6) and HR (control-vehicle, 347 ± 17.25, CsA, 368 ± 7.15 and Tac, 347 ± 6.48 BPM, n=5-6) between different groups (Figure 3).
Aortic contractile response
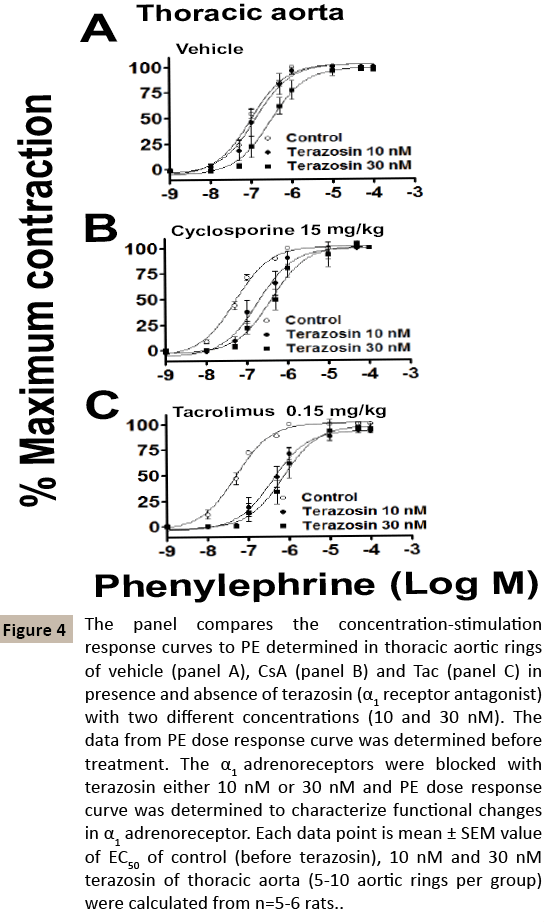
Later, both TA and AA were isolated and mounted in the organ bath. The contractility response to α1 agonist (PE-1 nM to 100 μM) was assessed in both TA and AA. In the TA, there was significant increase in contractility in CsA and Tac treated groups as compared to control-veh (Control-vehicle, EC50 104 ± 14.0 nM, CsA 55 ± 7.06 nM, Tac, EC50 50 ± 6.6 nM, p<0.01. n=5-6). On other hand, in the AA ring both CsA and Tac treated rats showed no alterations of contractile responses to PE (Control-vehicle, EC50, 74 ± 12.8 nM, CsA, EC50, 72 ± 7.60 nM, Tac, EC50, 59 ± 8.1 nM, n=5- 6) [Table 1-2 and Figure 3]. Further, we wanted to confirm the functional alterations caused by CsA and Tac to α1 adrenoreceptorinduced contractile mechanism, we used terazosin (selective α1 adrenoreceptor blocker) and PE-induced contractile response curve was derived against the two different concentrations (10 and 30 nM) of terazosin in both TA and AA (Figures 4 and 5). The dose ratio was calculated using EC50 of terazosin blockade to that of control EC50 (Table 2). The EC50 of control-veh PEinduced contraction in TA was 104 ± 14.0 nM and after 10 nM terazosin blockade it did not showed any difference 171 ± 56.3 nM. Whereas, after 30 nM terazosin blockade showed significant rightward shift of EC50 values (EC50, 362 ± 88.6 nM, p<0.05, n=5). Similarly, after CsA treatment there was no difference in terazosin 10 nM group as compared to EC50 without terazosin (Control EC50, 55 ± 7.06 nM; terazosin EC50, 231 ± 66.8 nM, n=6), but terazosin 30 nM group showed significant different in EC50 values 370 ± 79.7 nM vs. control). In other hand Tac treatment showed that there was significant difference in both terazosin 10 nM (control EC50, 50 ± 6.6 nM, vs terazosin 10 nM EC50, 433 ± 111.0 nM, p<0.05, n=5) and terazosin 30 nM (EC50, 1.04 ± 0.46 μM vs. control, p<0.01, n=5) blockade indication tacrolimus 0.15 mg/kg body wt. for 15 days might have caused significant α1 adrenoreceptor changes in TA leading to development of vasculature alterations. In contrast to the TA, the AA there were trend towards α1 adrenoreceptor alterations but there was no significant difference in EC50 of control and terazosin blockade of either 10 nM concentration, Control-vehicle (control-EC50, 74 ± 12.8 nM vs. terazosin-10 nMEC 50, 69 ± 20.5 nM, vs terazosin-30 nM-EC50, 207 ± 86.3 nM, n=5), CsA treatment (control-EC50, 72 ± 7.6 nM vs. terazosin-10 nMEC 50, 123 ± 49.0 nM, vs terazosin-30 nM-EC50, 373 ± 173.3 nM, n=6) and Tac treatment (control-EC50, 59 ± 8.1 nM vs terazosin-10 nM-EC50, 226 ± 75.5 nM vs terazosin-30 nM-EC50, 401 ± 98.3 nM, n=5, (Figures 4-7 and Table 2).
| Region | Treatment groups |
PE-Concentration response curve: Mean ± SEM value |
|
|---|---|---|---|
| EC50 | Emax (%) | ||
| Thoracic Aorta | Vehicle CsA (15 mg/kg) TAC (0.15 mg/kg) |
104 ± 14.0 nM 55 ± 7.06 nM** 50 ± 6.6 nM** |
100.0 ± 0.33 100.0 ± 0.31 99.9 ± 0.23 |
| Abdominal Aorta | Vehicle CsA (15 mg/kg) Tac (0.15 mg/kg) |
74 ± 12.8 nM 72 ± 7.60 nM# 59 ± 8.1 nM |
100.2 ± 0.20 100.0 ± 0.00 99.9 ± 0.13 |
Table 1: The comparative vascular contractility effects of two calcineurine inhibitors, cyclosporine (CsA, 15 mg/kg) and tacrolimus (Tac 0.15 mg/ kg) in SD rat after 15 days of treatment. The phenylephrine (PE)-induced contractility on thoracic and abdominal rat aortic rings with intact endothelium.
| Region | Treatment groups | Terazosin (T) | PE-concentration response curve (Mean ± SEM) | ||
|---|---|---|---|---|---|
| Emax (%) | EC50 | Concentration ratio (B-EC50/T-EC50) |
|||
| Thoracic Aorta | Vehicle | Before (B) | 100.1 ± 0.10 | 104 ± 14.0 nM | |
| (T) 10 nM | 99.8 ± 0.18 | 171 ± 56.3 nM | 1.65 | ||
| (T) 30 nM | 97.3 ± 2.18 | 362 ± 88.6 nM* | 3.50 | ||
| CsA (15 mg/kg) |
Before (B) | 100.5 ± 0.22 | 55 ± 7.06 nM | ||
| (T) 10 nM | 99.60 ± 0.40 | 231 ± 66.8 nM | 4.20 | ||
| (T) 30 nM | 100.4 ± 0.68 | 370 ± 79.7 nM* | 6.74 | ||
| Tac (0.15 mg/kg) |
Before (B) | 100.3 ± 0.31 | 50 ± 6.6 nM | ||
| (T) 10 nM | 98.1 ± 2.57 | 433 ± 111.0 nM* | 8.68 | ||
| (T) 30 nM | 97.0 ± 5.76 | 1.06 ± 0.46 µM** | 21.29 | ||
| Abdomin-al Aorta | Vehicle | Before (B) | 100.2 ± 0.25 | 74 ± 12.8 nM | |
| (T) 10 nM | 100.8 ± 0.49 | 69± 20.5 nM | 0.94 | ||
| (T) 30 nM | 100.6 ± 0.4 | 207 ± 86.3 nM | 2.80 | ||
| CsA (15 mg/kg) |
Before (B) | 100.4 ± 0.22 | 72±7.6 nM | ||
| (T) 10 nM | 100.6 ± 0.40 | 123± 49.0 nM | 1.70 | ||
| (T) 30 nM | 100.2 ± 0.49 | 373 ± 173.3 nM | 5.15 | ||
| Tac (0.15 mg/kg) |
Before (B) | 99.9 ± 0.13 | 59±8.1 nM | ||
| (T) 10 nM | 100.5 ± 0.50 | 226± 75.5 nM | 3.85 | ||
| (T) 30 nM | 101.0 ± 0.41 | 401 ± 98.3 nM | 6.82 | ||
Table 2: Action of two calcineurine inhibitors, CsA (15 mg/kg) and Tac (0.15 mg/kg) on vascular reactivity when treated in SD rat for 15 days. The assessment of contractility was done using α1 adrenergic receptor blockers against the phenylephrine-induced dose response curve.
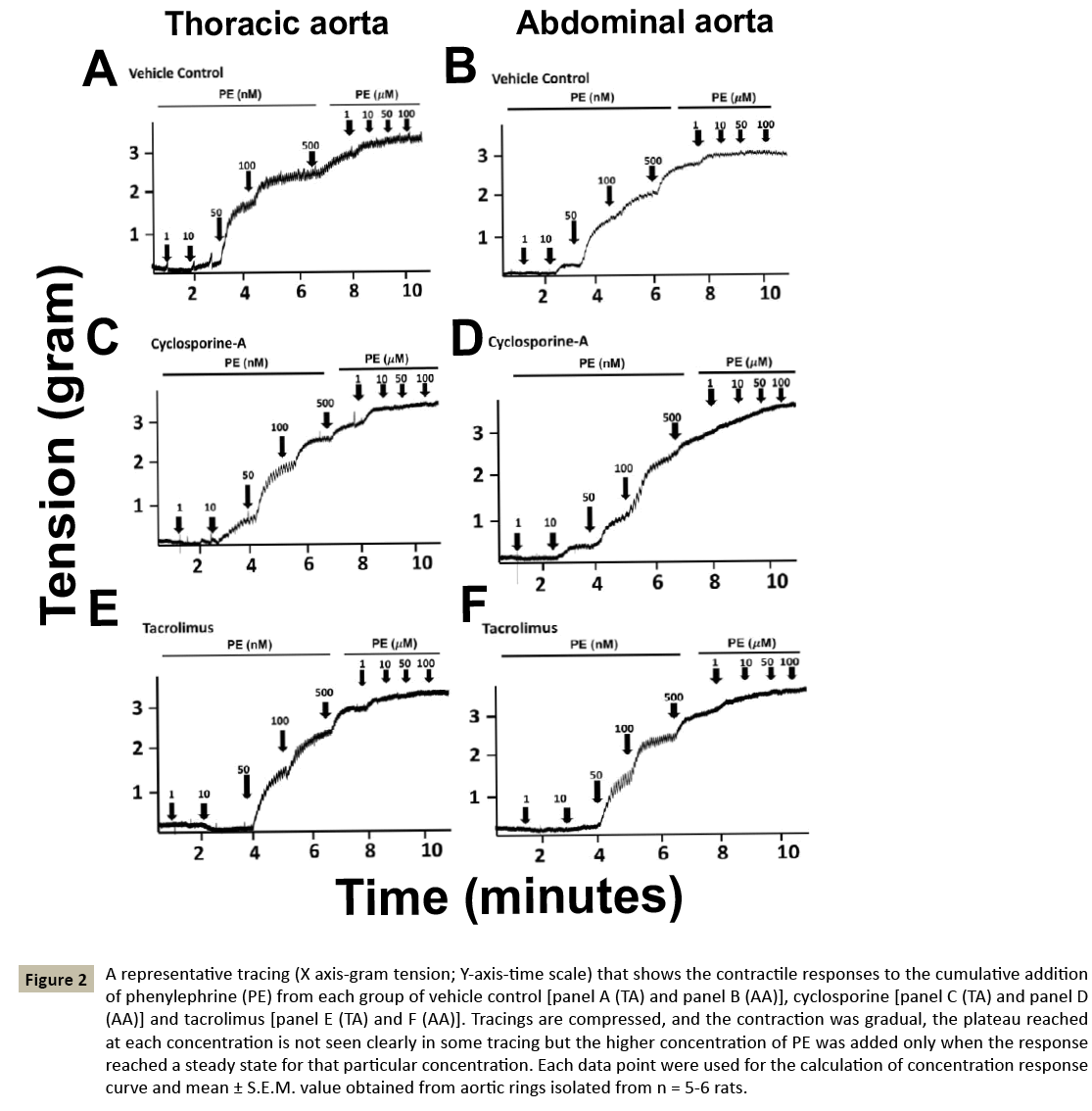
Figure 2: A representative tracing (X axis-gram tension; Y-axis-time scale) that shows the contractile responses to the cumulative addition of phenylephrine (PE) from each group of vehicle control [panel A (TA) and panel B (AA)], cyclosporine [panel C (TA) and panel D (AA)] and tacrolimus [panel E (TA) and F (AA)]. Tracings are compressed, and the contraction was gradual, the plateau reached at each concentration is not seen clearly in some tracing but the higher concentration of PE was added only when the response reached a steady state for that particular concentration. Each data point were used for the calculation of concentration response curve and mean ± S.E.M. value obtained from aortic rings isolated from n = 5-6 rats.
Figure 3: The panel compares the concentration-stimulation response curves to phenylephrine (PE) 1 nM to 100 μM determined in thoracic (A) and abdominal (B) aortic rings with intact aortic rings. Rats were treated with vehicle (castor oil, s/c), CsA (15 mg/kg, s/c) and Tac (0.15 mg/ kg, s/c) for 15 days. End of the study, both thoracic and abdominal aortas were isolated and 3 mm aortic rings were prepared and mounted in organ bath and dose response curve were plotted against PE. Each data point is mean ± SEM value obtained from aortic rings (8-12 aortic rings per group) isolated from n=5-6 rats.
Figure 4: The panel compares the concentration-stimulation response curves to PE determined in thoracic aortic rings of vehicle (panel A), CsA (panel B) and Tac (panel C) in presence and absence of terazosin (α1 receptor antagonist) with two different concentrations (10 and 30 nM). The data from PE dose response curve was determined before treatment. The α1 adrenoreceptors were blocked with terazosin either 10 nM or 30 nM and PE dose response curve was determined to characterize functional changes in α1 adrenoreceptor. Each data point is mean ± SEM value of EC50 of control (before terazosin), 10 nM and 30 nM terazosin of thoracic aorta (5-10 aortic rings per group) were calculated from n=5-6 rats.
Figure 5: The panel compares the concentration-stimulation response curves to PE determined in abdominal aortic rings of vehicle (panel A), CsA (panel B) and Tac (panel C) in presence and absence of terazosin with two different concentration (10 and 30 nM). The PE dose response curve was determined before treatment. The α1 adrenoreceptors were blocked with terazosin either 10 nM or 30 nM and PE dose response curve was determined to characterize functional changes in α1-adrenoreceptor. Each data point is mean ± SEM value of EC50 of control (before terazosin), 10 nM and 30 nM terazosin of abdominal aorta (5-8 aortic rings per group) were calculated from n=5-6 rats.
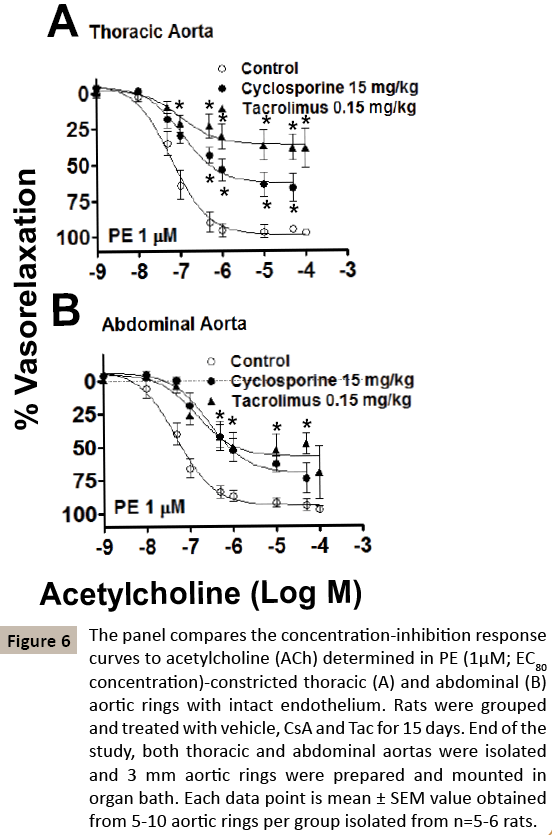
Figure 6: The panel compares the concentration-inhibition response curves to acetylcholine (ACh) determined in PE (1μM; EC80 concentration)-constricted thoracic (A) and abdominal (B) aortic rings with intact endothelium. Rats were grouped and treated with vehicle, CsA and Tac for 15 days. End of the study, both thoracic and abdominal aortas were isolated and 3 mm aortic rings were prepared and mounted in organ bath. Each data point is mean ± SEM value obtained from 5-10 aortic rings per group isolated from n=5-6 rats.
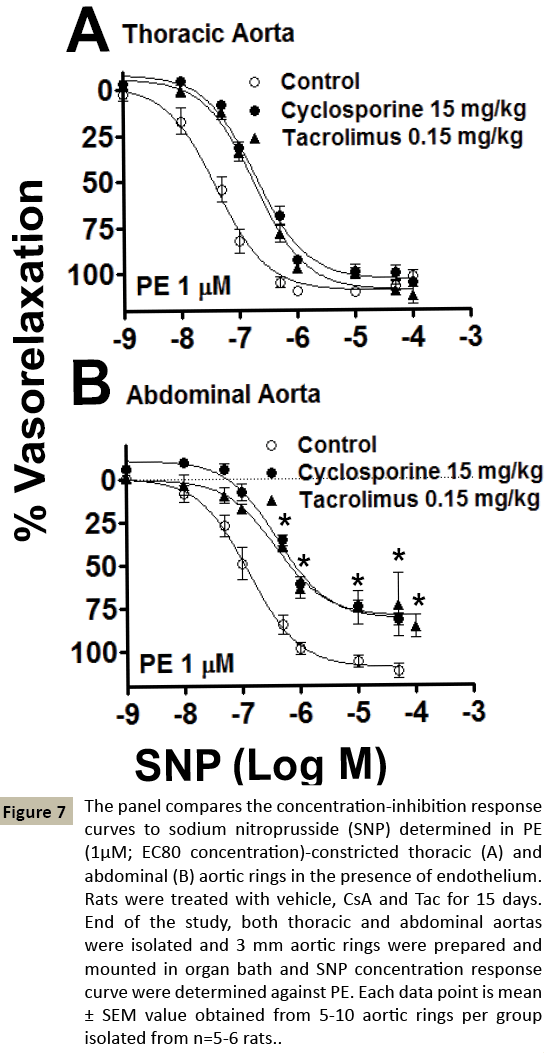
Figure 7: The panel compares the concentration-inhibition response curves to sodium nitroprusside (SNP) determined in PE (1μM; EC80 concentration)-constricted thoracic (A) and abdominal (B) aortic rings in the presence of endothelium. Rats were treated with vehicle, CsA and Tac for 15 days. End of the study, both thoracic and abdominal aortas were isolated and 3 mm aortic rings were prepared and mounted in organ bath and SNP concentration response curve were determined against PE. Each data point is mean ± SEM value obtained from 5-10 aortic rings per group isolated from n=5-6 rats.
Aortic endothelial-dependent relaxation
The endothelium- dependent (ACh) vascular relaxation response curves were obtained using ACh, a muscarinic receptor agonist, in both PE-induced constricted TA and AA. As these aortic rings were collected from the rat treated with vehicle, CsA and Tac after 15 days of treatment, and (Mean ± SEM) IC50 and Emax values were compared between the groups. It is clear that TA from Tactreated rats showed severe endothelium-dependent impaired relaxation as compared to CsA treated rats (Control-veh, IC50 99 ± 32.3 nM, CsA-IC50, 239 ± 84.0 nM, Tac-IC50, 511 ± 84.0 nM, non-significant, n=5; Control-Emax, 98 ± 2 %, CsA-Emax, 66 ± 8.79 %, Tac-Emax, 39 ± 9.55 %, p<0.01, n=5-6). The severity of impaired vascular relaxation in AA was less compared to TA (Control-veh, IC50 57 ± 15.6 nM, CsA-IC50, 442 ± 140 nM, Tac-IC50, 169 ± 47.5 nM, p<0.05, n=5; Control-Emax, 97 ± 2.6 %, CsA-Emax, 74 ± 11.38 %, Tac- Emax, 70 ± 20.06 %, p<0.05, n=5-6).
Aortic endothelial-independent relaxation
The endothelium-independent vascular smooth relaxations were assessed using SNP. The endothelium-independent impaired vascular relaxations were comparable in both CsA and Tac treated rats in both TA (Control-veh-IC50 47 ± 11.2 nM, CsA IC50, 214 ± 27.9 nM, Tac-IC50, 191 ± 30.8 nM, p<0.01, n=5; Control-Emax, 101 ± 2.84 %, CsA-Emax, 105 ± 1.45 %, Tac-Emax, 112 ± 3.28 %, n=5-6) and AA (Control-veh-IC50 164 ± 45.1 nM, CsA-IC50, 424 ± 53.9 nM, Tac-IC50, 520 ± 104.1 nM, p<0.01, p<0.05, n=5; Control-veh-Emax, 111 ± 3.29 %, CsA-Emax, 82 ± 3.55 %, Tac-Emax, 86 ± 4.56 %, p<0.05, n=5-6). Overall, our functional data shows that there was vascular alteration in both aortic sections of TA and AA, by CNIs treatment. The differential effects of both CsA and Tac was noted differently either by alteration of α1 adrenoreceptor or by impairment of vascular smooth muscle relaxation via endothelium-dependent or independent mechanism. This might be linked with downregulation of nitric oxide production as well as α1 adreno-receptor structural changes.
Discussions
The present study is the first investigation to determine the in vivo effects of CNIs, CsA and Tac on different portions of rat aortic tissue (TA and AA) contractility, and both endothelium-dependent and endothelium-independent relaxation using same rat tissues. The treatment doses of both CsA and Tac were used in rats are from the human therapeutic dose range. However administration of drugs were done subcutaneously. The data we have gathered are in the relevant only in the context of the present experimental models and doses administered s/c in the SD male rat [27]. In our pilot study, we treated rats with a dose of CsA, 15 mg/kg, Bwt for 10 days, but we did not found any changes with respect to haemodynamic parameter as well as vascular injury in rat aorta (unpublished data).Therefore, in the current study we decided to extend the period of treatment for 2 weeks, s/c injections which yielded vascular damage. Several researchers have used different doses of CsA ranging from 5 mg/kg Bwt to 50 mg/kg Bwt., with a treatment period ranging from 7 days to 45 days in rats and or rabbit (18, 19, 20). Although, previous studies have used Tac doses between 0.5 to 5 mg/kg Bwt. orally for up to 4 weeks [28], in our study we decided to use 0.15 mg/kg Bwt, s/c on the basis of its potency (10-100 times more) as compared to CsA (15). Arterial BP were within normal range in rats treated with vehicle. Both CsA and Tac treatment showed increase in the arterial BP as compared to control-vehicle group. Our finding of significant higher BP in the Tac group was consistent with previous study with treatment of Tac, 1mg/kg, 15 days s/c injections in the rodent model [29]. In the normotensive rat model, CsA treatment showed transient increase in BP. It is well known that in normal rat CsA may increase [30], or decrease transiently [31] and not affect [9] arterial BP [32]. In our study, other BP parameters are the reflections of arterial BP, which were calculated by using cyclic measurement tool on Lab Chart 5 program. In these parameters, as compare to vehicle control group, diastolic BP was significantly higher in Tac but not CsA group. It may be linked with the arterial stiffness caused due to Tac reflecting higher diastolic BP (33). CsA do has more effects on arterial stiffness than Tac, but it was not reflected in our study [33]. The effect of CsA on adrenergic vascular reactivity has been studied widely but none of the literatures available for Tac. Surprisingly, no one has used terazosin as a α1-adrenoreceptor blocker for the characterization of vascular reactivity of CNI. Recently Bergler T, et al., showed that CsA treatment causes marked reduction in nonepinephrineinduced contractile response of mesenteric resistance arteries, while there were no changes in contraction by endothelin. Decrease in contractility was accompanied by marked downregulation of adrenoreceptor in mesenteric resistance arteries [34]. These results encouraged us to design as study to characterize differential vascular contractility of both CsA and Tac in the in vivo rat model and compare them by using other vessels such as TA and AA. These aortae are well known for the existence of α1-adrenergic adrenoreceptors in the rats [35]. In our study, the vascular contractility was assessed by using PE, a selective α1- adrenergic receptor agonist. Which consistently behaved as a full agonist by showing maximum contractile effects on both rat TA and AA [36]. In addition, a selective α1-adrenergic receptor blocker terazosin was utilised and we found that it was competitive in both TA and AA. Although we have not used 3 different concentrations of terazosin to calculate pA2 (antagonist potency) values due to utilization of portion of vascular rings for some other experiments such as vascular relaxation, we still managed to use 2 concentrations of terazosin to calculate concentration ratio from PE-contracted TA and AA. The control groups showed concentration ratio values, which are around the same range of the previously reported concentration ratio data, which were further calculated to yield functional affinity value (pA2) against the contraction to noradrenalin in rat aorta [37,38,39]. In present study, results showed that CsA and Tac treatment enhanced vascular contractibility in the TA but not in the AA. It may be linked with segmental difference in α1-adrenoreceptor subtype in rat abdominal and thoracic aortae [40]. The effect of CNIs on vasculature were due to its action on inhibition of endotheliumdependent NO-induced vasorelaxations [8,41,42]. Although, NOinduced vascular actions is important for vascular homeostasis, vascular noradrenalin receptors also plays important role in the regulation of vascular tone. CsA increases vascular contraction by stimulating nor-adrenaline in rat aorta [43]. The increase in PEinduced contractions was noted in rabbit renal artery at the concentration of 1-10 μM of cyclosporine without changing pD2 values [44] and increase in vascular sensitivity to adrenergic agonists in the presence of CsA which was also showed by some researcher [45]. Other studies have also demonstrated that CsA reduces vascular sensitivity to nor-adrenaline without change in maximal contractility [44] as well as aortic rings response to PE [46]. Surprisingly, only one study showed the vascular contractility by Tac, which was done in the TA but not in the AA [27]. Treatment with CsA and Tac may produce reactive oxygen species [27,46], which may further damages an α1-adrenoreceptor and alter the functional activity of terazosin. These alteration of terazosin activity was predominantly noted in the TA but not in the AA indicating differential effects of CsA and Tac at different portion of rat aorta. Our second objective was to characterize vascular relaxation affected by both CsA and Tac, therefore we used AChinduced endothelium-dependent as well as SNP-induced endothelium-independent vascular relaxation pathway. As we know that ACh-induced endothelium-dependent vasodilatation is mediated solely by the release of NO in major conduit vessels such as TA [47,48]. Whereas, 3 different endothelium dependent vasodilator pathways such as NO, endothelium-derived hyperpolarization factor (EDHF) and/or prostacyclin (PGI2) are present in smaller vessels such as descending AA, secondary mesenteric artery and their branches [48-53]. In our study we found that both CsA and Tac showed blunting effect on AChinduced vasodilatation in the TA as compared to AA. It is directly linked with their selective blocking action on eNOS expression via alteration with serine threonine phosphorylation sites on NOS [54]. In case of AA, CNIs selectively inhibit only eNOS, but not other vasodilatory pathways EDHF and PGI2. In current study, as compare to CsA, Tac has profound inhibition effect on AChinduced vasodilatation, which is contradicting previous finding that CsA affect more than the Tac on the vascular functional capacity [27,55]. In the clinical settings, the incidence of hypertension has been higher following CsA treatment as compared to Tac [1,5], while Tac has higher incidences of nephrotoxicity and neuotoxicity [1,5]. Although most of the research showed that CsA is more potent for the vascular damages than Tac, there are some report also showed that supraclinical and clinical doses of tacrolimus is associated with vascular endothelium dysfunction in both animal models and human transplant recipients [27,40,55,56]. In addition, Can et.al., found that when wistar rat treated with Tac for 14 days caused impaired relaxation response to ACh [57]. Other in vitro finding was that the incubation of Tac with mouse aorta in organ bath showed impaired endothelial-dependent relaxation response to ACh which was linked to altered intracellular calcium release affecting eNOS [58]. Our present study supports all these findings with the Tac-induced vascular endothelium dysfunction. Endothelium-independent vascular relaxation was assessed using SNP-induced vascular relaxation which is most common in vitro technique used in the vascular pharmacology. In current study we found that in the TA, CNIs shifted SNP-induced Concentration response curve rightward with restoring of Emax indicating there are some vascular smooth muscle damages. However, in the AA both CsA and Tac showed rightward shift with reduced Emax confirms the intensity of damages are more as compared to the TA. These findings are consistent with previous study which found Tac inhibits the SNP-induced vascular relaxation with reduced Emax [27]. But the vascular dysfunction caused between TA and AA may be linked with the differences in vascular relaxation pathways. Recently, we know that the TA is predominantly relaxed by NO indicating TA vascular smooth muscles do contain NO sensitive cGMP enzymes as compared to the AA. While AA, besides NO sensitive cGMP, there may be more prostaglandin induced as well as EDHF sensitive calcium channels are involved to cause vascular relaxations. Vascular dysfunction caused by CNIs has implication for the development of hypertension and intern risk of cardiovascular disease. It is important to assess different doses of immunotherapy agents as they shows different potencies and efficacy. As it is fully established that Tac is 10-100 more potent than CsA. That means Tac at 100 times lower concentration of CsA might cause vascular dysfunction which is one of the side effect of these immunotherapeutic agents. The vascular dysfunction either comes from enhanced vascular contractibility or reduced vascular relaxation capacity of the vessels. Therefore, in the present study we strived to measure functional parameters of contractibility and relaxations using same animal model, same dosing conditions and same in vitro organ bath techniques after 15 days of CNIs administrations. We think our present study may prove valuable information for the field of post-transplant immunotherapy.
Acknowledgments
This study was supported by Dr. Ahmed Shoker through the Renal Transplant Program, Division of Nephrology, Department of Medicine, Royal University Hospital, Saskatoon, Canada.
References
- Stepkowski SM (2000) Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med 2:1-23.
- Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, et al. (2012) Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol 25:269-275.
- Benett WM (1995)The nephrotoxicity of immunosuppressive drugs.ClinNephrol 1:S3-S7.
- Textor SC (1993) De-novo hypertension after liver transplantation. Hypertension 22:257-267.
- The US Multicenter FK506 Liver Study Group. (1994) A comparison of tacrolimus for immunosuppression in liver transplantation. N Eng J Med 331:1110-1115.
- Sturrock ND, Lang CC, Struthers AD (1993) Cyclosporine-induced hypertension precedes renal dysfunction and sodium retention in man, J Hypertens 11:1209-1216.
- Golbaekdal K, Nielsen CB, Pedersen EB (1996) The acute effects of FK-506 on renal haemodynamics, water and sodium excretion and plasma levels of angiotensin II, aldosterone, atrial nitriuretic peptide and vasopressin in pigs. J Pharm Pharmacol 48:1174-1179.
- Textor SC, WiesnerR, Wilson DJ, Porayko M, Romero JC, et al. (1993) Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation55:1332-1339.
- Textor SC, Smith-Powell C, Telles T (1990) Altered pressor response to NE and ANG II during cyclosporine A administration to conscious rats. Am J Physiol 258:H854-H860.
- Verbeke M, Van de Voorde, J, Kesteloot D, Waterloos M, De Ridder L, et al. (1992) Vascular reactivity in cyclosporine (Cs)-nephrotoxicity: a morphological and functional study. Kidney intern 41: 1432.
- Verbeke M, Van de Voorde J, Lameire N (1993) Functional study of cyclosporine-induced renal vasculotoxicity on rats. In Proceeding of the 3rd Satellite Symposium on ARF, Halkidiki.Papadimitriou M,AlexopoulosE, Univesity Studio Press, Thessaloniki, Greece.
- Schnabel FR, Wait RB, Aaronson O,Kahng KU (1989) Effect of cyclosporine administration on vascular reactivity in the rabbit. Transplant Proc 21:918-921.
- Takenaka T, Hashimoto Y, Epstein M (1992) Diminished acetylcholine-induced vasodilatation in renal microvessels of cyclosporine-treated rats. J Am SocNephrol 3:42-50.
- Auch-Schwelk W, Bossaller C, Gotze S, Thelen J, Fleck E (1993) Endothelial and vascular smooth muscle function after chronic treatment with cyclosporine A. J Cardiovascular Pharmacology 21:435-440.
- Verbeke M, Van de Voorde J, De Ridder L, Lameire N (1994) Functional analysis of vascular dysfunction in cyclosporine-treated rats. Cardiovasc Res 28:1152-1156.
- Diedrich D, Yang Z, Luscher F (1992) Chronic cyclosporine therapy impairs endothelium-dependent relaxation in the renal artey of the rat. J Am SocNephrol 2:1291-1297.
- Mikkelsen EO, Poulsen SH, Nyborg NCB, Korsgaard N, Sehested J (1992) Difference between aortic and renal vascular reactivity in cyclosporine A treated rats and the effect of cicletanine. Naunyn-Schmiedeberg’s Arch Pharmacol 345:356-361.
- Roullet JB, Xue H, McCarron D, Holcomb S, Bennett WM (1994) Vascular mechanisms of cyclosporine-induced hypertension in the rat. J Clin Invest 93:2244-2250.
- Gallego MJ, Garcia Villalon AL, Lopez Farre AJ, Garcia JL, Placida Garron M, et al. (1994) Mechanisms of the endothelial toxicity of cyclosporin A . Circ Res 74:477-484.
- VerbekeM, Van de Voorde J, Ridder de L, Lameire N (1995) Regional differences in vasculotoxic effects of cyclosporine in rats. Can J PhysiolPharmacol 73:1661-1668.
- Yaris E, Tuncer M (1995) Cyclosporine A and Cremophor-EL augment renal vascular responses to various agonists and nerve stimulation. Arch IntPharmacolTher 329:405-417.
- Lamb FS, Webb RC (1987) Cyclosporine augments reactivity of isolated blood vessels. Life Sci 40:2571-2578.
- Jadhav A, Gopalakrishnan V, Shoker A (2013) Comparative In VitroEffects of Calcineurin Inhibitors on Functional Vascular Relaxations of Both Rat Thoracic and Abdominal Aorta.Advances inPharmacological Sciences.
- Jadhav A, Liang W, Balsevich J, Bastin G, Kroetsch J, et al. (2012) L-tryptophan ethyl ester dilates small mesenteric arteries by inhibition of voltage-operated calcium channels in smooth muscle. Br J Pharmacol 166: 232-242.
- Jadhav AB, Liang W, Papageorgiou PC, Shoker A, Kanthan SC, et al. (2013)Catharanthine dilates small mesenteric arteries and decreases cardia contractibility by inhibition of voltage-operated calcium channels. J PharmacolExpTher 345: 383-392.
- Hopfner RL, Hasnadka RV, Wilson TW, McNeill JR, Gopalakrishnan V (1998) Insulin increases endothelin-1-evoked intracellular free calcium responses by increased ET(A) receptor expression in rat aortic smooth muscle cells. Diabetes 47: 937-944.
- Shing CM, Fassett RG, Brown L, Coombes JS (2012)The effects of immune-suppressants on vascular function, systemic oxidative stress and inflammation in rats. Transplant International 25: 337-346.
- Roullet JB, Xue H, McCarron DA, Holcomb S, BennettWM (1994) Vascular mechanisms of cyclosporine-induced hypertension in the rat. J Clin Invest 93:2244-2250.
- Hoorn JE, Walsh SB, McCormicki JA, Fürstenberg A, Yang CL, et al. (2011)Thecalcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17:1304-1309.
- Lusting S, Stern N, Golub MS, Eggena P, Barrett J, et al. (1989) Experimental cyclosporin hypertension: characterization of the rat model. Transplant. Proc 21: 950-951.
- Murray BM, PallerMS, FerrisTF (1985) Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney int 28: 767-774.
- RegoA, Vargas R, Cathapermal S, Kuwahara M, Foegh M,et al. (1991) Systemic vascular effects of cyclosporine A treatment in normotensive rats. J Pharmacol Exp The r259:905-915.
- Seibert F, BehrendtC, Schmidt S, Giet MVD, Zidek W, et al. (2011) Differential effects of cyclosporine and tacrolimus on arterial function. Transplant Int 24: 708-715.
- Bergler T, Resch M, Reinhold SW, Birner C, Jungbauer CG, et al. (2012)Cyclopsorine A impairs norepinephrine-induced vascular contractility. Kidney Blood Press Res 35:655-662.
- Saussy DL, Goetz AS, Queen KL, King HK, Lutz MW, et al. (1996) Structure activity relationships of series of buspirone analogs at alpha-1 adrenoceptors:further evidence that rat aorta alpha-1 adrenoceptors are of the alpha-1D subtype. J PharmacolExpTher 278:136-144.
- Buckner SA, Oheim KW, Morse PA, Knepper SM, Hancock AA (1996) a1-adrenoceptor-mediated contractility in rat aorta is mediated by the a1D subtype. Eur J Pharmacol 297:241-248.
- Aboud R, Shafil M, Docherty JR (1993) Investigation of the subtypes of a1adrenoceptor mediating contraction of rat aorta, vas deferens and spleen. Br J Pharmacol 109:80-87.
- Kenny BA, Chalmers DH, Philpott PC, Naylor AM (1995) Characterization of alpha 1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol 115: 981-986.
- Testa R, Destafani C, Guarneri L, Poggesi E, Simonazzi I, et al. (1995)The a1d-adrenoceptor subtype is involved in the noradrenalin-induced contractions of rat aorta. Pharmacol. Lett57: PL159-PL163.
- Asbun-Bojalil J, Castillo EF, Escalante BA, Castillo C (2002) Does segmental difference in a1-adrenoceptor subtype explain contractile difference in rat abdominal and thoracic aortae?Vascular Pharmacology 38:169-175.
- Takeda Y, Yoneda T, Ito Y, Miyamori I, Takeda R (1993) Stimulation of endothelin mRNA and secretion in human endothelial cells by FK 506. J CardiovascPharmaco 22: S310:622.
- Takeda Y, Miyamori I, Furukawa K, Satoru I, Mabuchi H (1999) Mechanisms of FK 506-Induced Hypertension in the Rat. Hypertension 33:130-136.
- Yaris E, Tuncer M (1995) Cyclosporine A and Cremophor-EL augment renal vascular responses to various agonists and nerve stimulation. Arch IntPharmacolTher 329:405-417.
- Lamb FS, Webb RC (1987) Cyclosporine augments reactivity of isolated blood vessels. Life Sci 40:2571-2578.
- Waldman SA, Murad F (1987) Cyclic GMP synthesis and function. Pharmacol Rev 39:163-196.
- Gao YJ, Lee RM (2005) Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br. J Pharmacol 146: 1061-1068.
- Agnew AJ, Robinson E, McVicar CM, Harvey AP, Ali IH, et al. (2012) The gastrointestinal peptide obestatin induces vascular relaxation via specific activation of endothelium-dependent NO signalling. Br J Pharmacol 166: 327-338.
- Zhang R, Ran HH, Zhang YX, Liu P, Lu CY, et al. (2012) Farnesoid X receptor regulates vascular reactivity through nitric oxide mechanism. J PhysiolPharmacol 63: 367-372.
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, et al. (2002) EDHF: bringing the concepts together. Trends PharmacolSci 23: 374-380.
- Bobadilla RA, Henkel CC, Henkel EC, Escalante B, Hong E (1997) Possible involvement of endothelium-derived hyperpolarizing factor in vascular responses of abdominal aorta from pregnant rats. Hypertension 30: 596-602.
- Tomioka H, Hattori Y, Fukao M, Sato A, Liu M, et al. (1999) Relaxation in different-sized rat blood vessels mediated by endothelium-derived hyperpolarizing factor: importance of processes mediating precontractions. J Vasc Res 36: 311-320.
- Woodman OL, Wongsawatkul O, Sobey CG (2000) Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries. ClinExpPharmacolPhysiol 27: 34-40.
- Kwan CY, Zhang WB, Sim SM, Deyama T, Nishibe S (2004) Vascular effects of Siberian ginseng (Eleutherococcussenticosus): endothelium-dependent NO- and EDHF-mediated relaxation depending on vessel size. NaunynSchmiedebergs Arch Pharmacol 369: 473-480.
- Lungu AO, Jin ZG, Yamawaki H, Tanimoto T, Wong C, et al. (2004) Cyclosporin A inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J BiolChem 279: 48794-48800.
- Galle J, Lehmann-Boden C, Hubner U, Heinloth A,Wanner C (2000) CyA and OxLDL cause endothelial dysfunction in isolated arteries through endothelin-mediated stimulation of O(2)(-) formation. Nephrol Dial Trasplant 15: 339-346.
- Oflaz H, Turkmen A, Kazancioglu R, Kayacan SM, Bunyak B, et al. (2003) The effect of calcineurin inhibitors on endothelial function in renal transplant recipients. ClinTrasplant 17:212-216.
- Can C, Erol A, Cinar M, Olukman M, Ulker S, et al. (2007) Therapeutic concentrations of tacrolimus do not interfere with endothelial nitric oxide synthesis in rat thoracic aortas and coronary arteries. J Cardiovasc Pharmacol 50:399-405.
- Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM (2009)Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int 75:719-726.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences