A review on: Biological activities, SAR of pyrimidine with marketed drug
Vaishnavi Mohite*, Suchitra Patil, Shubhangi Shelke, Vaishnavi Adake, Pramod Patil and Sachinkumar Patil
Department of Pharmaceutical Quality Assurance, Ashokrao Mane College of Pharmacy, PethVadgaon, Maharashtra, India
Published Date: 2023-03-21Vaishnavi Mohite*, Suchitra Patil, Shubhangi Shelke, Vaishnavi Adake, Pramod Patil and Sachinkumar Patil
Department of Pharmaceutical Quality Assurance, Ashokrao Mane College of Pharmacy, PethVadgaon, Maharashtra, India
- *Corresponding Author:
- Vaishnavi Mohite
Department of Pharmaceutical Quality Assurance,
Ashokrao Mane College of Pharmacy,
PethVadgaon, Maharashtra,
India
Tel: 9767621708
E-mail: vaishnavimohite2015@gmail.com
Received date: January 12, 2023, Manuscript No. IPJSVP-23-15633; Editor assigned date: January 16, 2023, PreQC No. IPJSVP-23-15633 (PQ); Reviewed date: January 31, 2023, QC No. IPJSVP-23-15633; Revised date: March 13, 2023, Manuscript No. IPJSVP-23-15633 (R); Published date: March 21, 2023, DOI: 10.36648/2469-6692.9.1.003
Citation: Mohite V, Patil S, Shelke S, Adake V, Patil P, et al. (2023) Review on: Biological Activities, SAR, Synthesis of Pyrimidine with Marketed Drug. In Silico In Vitro Pharmacol Vol:9 No:1
Abstract
Pyrimidines have a significant role in medicine according to their wide range of biological activities. They are of interest as possible bioactive compounds along with their related fused heterocycles. The pharmacological effects of pyrimidine derivatives have been reported to include anticonvulsant, analgesic, sedative, antidepressant, antipyretic, anti-inflammatory, antiviral, anti-HIV, antibacterial, and anti-tumour effects. As a result, the focus of the research presented in this review has been on studies that have been published in recent scientific literature and that examine various biological activities of pyrimidine analogues. This is the first review to in-depth discuss the pyrimidine and its substituted derivatives. A number of methods for the synthesis of pyrimidines have been documented, along with some of their biological activity.
Keywords
Pyrimidine; Pyrimidine derivatives; Marketed drug; Antibacterial; Antioxidant; Antifungal; Antiviral
Introduction
The biological significance of the pyrimidine derivatives has led us to the synthesis of substituted pyrimidine [1]. Pyrimidine is a fundamental component in DNA and RNA, it has been discovered to be connected to a variety of biological processes. Numerous in-depth reviews and the synthesis of substituted pyrimidine have been published. Acetyl acetone serves as a great illustration because it reacts well with formamidine, guanidine, urea, or thiourea to make the appropriate 4,6-dimethyl pyrimidine. The nitrogen-containing fragment may be an amidine, urea, thiourea or guanidine. Pyrimidines and their derivatives are regarded as crucial ingredients in pharmaceuticals and agricultural chemicals. Pyrimidine compounds have a variety of intriguing biological properties, including antibacterial, antitumor, and antifungal properties [2]. Leukaemia treatments and thyroid medications both involve a lot of pyrimidine derivatives.
Literature Review
Pyrimidine is a member of a heterocycle with a lot of electrons and nitrogen. The pyrimidine's adaptability in synthetic processes enables the creation of structurally diverse derivatives, such as analogues produced by substituting the aryl ring, derivatizing the pyrimidine's nitrogen, and substituting carbon at the positions 2, 4, 5, and 6 [3].
The various intrinsic biological characteristics pyrimidines are utilised as Mycobacterium tuberculosis, antioxidants, and antidiabetics, making them appealing to researchers in the field of pharmaceutical chemistry [4]. Pyridopyrimidine-containing heterocycles offer anti-allergic, antioxidant, antiviral, antihistaminic, cytostatic, immunomodulating, herbicidal, and anticonvulsant medicinal effects. The antimicrobial properties of pyrimidines include fungicidal, antitoxoplasma, antimalarial, antibacterial, antifilarial and antileishmanial effects.
Pyrimidine nuclei function as antibacterial agents, making them crucial molecules in food production and pharmaceuticals [5]. Pyrimidines are a significant and fascinating class of antibacterial medications that, especially in the last few years, have had a significant impact on the antibacterial chemotherapy area. The pyrimidine nucleus has anticancer and chemotherapeutic properties.
At least half of organic chemistry research is done on heterocyclic compounds. Many pharmacological, agricultural and veterinary medicines, in particular, are based on heterocyclic compounds. They come from a group of substances having demonstrated practicality in medicinal chemistry [6]. There are lots of six-membered rings with two hetero atoms, biologically active compounds. Pyrimidine is a heterocyclic aromatic chemical molecule with two nitrogen atoms at positions 1 and 3 of the six-member ring, comparable to benzene and pyridine (Table 1) [7].
| Antibacterial | |||
| Trimethoprim | 5-Bromo-2’-deoxyuridine; 5-Bromo-1-(2-deoxy-β-d-ribofuranosyl) pyrimi dine-2,4(1H,3H)-dione | Broxuridine has been used in trials studying the treatment of leukemia, stage I prostate cancer, stage IIB prostate cancer, and stage IIA prostate cancer. | 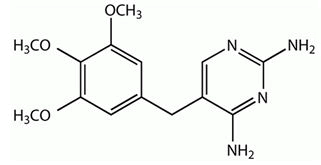 |
| Piromidic acid | 8-Ethyl-5,8-dihydro- 5-oxo-2-(pyrrolidin-1-yl) pyrido [2,3-d] pyrimidine-6- carboxylic acid | Piromidic acid is used in the treatment of urinary tract and intestinal infections. Antibacterial against mainly gram-negative organisms. | 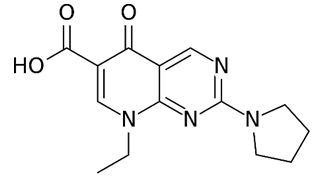 |
| Tetroxoprim | 5-[3,5-Dimethoxy-4- (2-methoxyethoxy)benz yl] pyrimidi ne-2,4-diyl-diamine | Tetroxoprim is an inhibitor of bacterial dihydrofolate reductase. It has been used for the treatment of susceptible bacterial infections. | 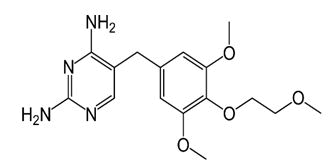 |
| Metioprim | 5-(3,5-Dimethoxy-4-methylthiobenzyl-2),4, | Trimethoprim is an antibiotic. It's used to treat and prevent Urinary Tract Infections (UTIs), such as cystitis. | 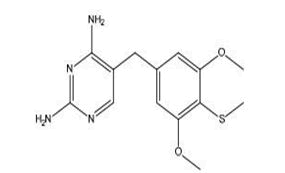 |
| Antifungal | |||
| Flucytosine | 5-Fluorocytosine; 4-Amino-5-fluoropyrimidine-2(1H)-one | Flucytosine is a medication used in the management and treatment of systemic and severe candida flucytosine is a medication used in the management and treatment of systemic and severe candida and cryptococcus infections | 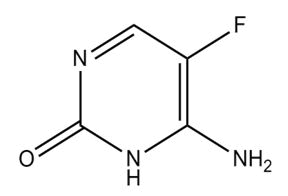 |
| Antivirals | |||
| Broxuridine | 5-Bromo-2’-deoxyuridine;5-Bromo-1-(2-deoxy-β-d-ribofuranosyl)pyrimidine-2,4(1H,3H)-dione | Broxuridine has been used in trials studying the treatment of leukemia, stage I prostate cancer, stage IIB prostate cancer, and stage IIA prostate cancer. | 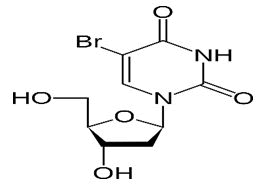 |
| Idoxuridine | 2’-Deoxy-5-iodouridine | Idoxuridine slows the growth of viruses that cause certain eye infections. | 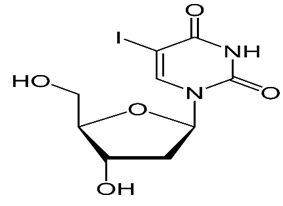 |
| Anthelmintic | |||
| Pyrantel embonate | 1,4,5,6-Tetrahydro-1-methyl-2-[(E)-2-(2-thienyl) vinyl] pyrimidine 4,4’-methylenebis(3-hydroxy-2-naphthoate) | This medication is used to treat intestinal worm infections such as pinworm, roundworm, and hookworm. | 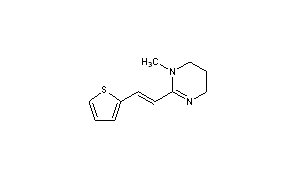 |
| Vasodilators | |||
| Trapidil | 7-Diethylamino-5- methyl-1,2,4- triazolo [1,5-] pyrimidine | Trapidil, a platelet derived growth factor antagonist, was originally developed as a vasodilator and anti-platelet agent and has been used to treat patients with ischemic coronary heart, liver, and kidney disease. | 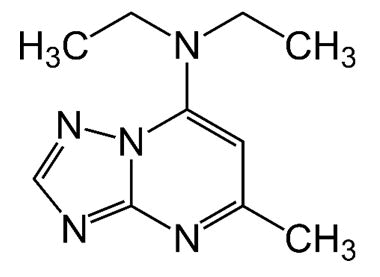 |
| Liver disorder | |||
| Orotic acid | 1,2,3,6-Tetrahydro-2,6-dioxopyrimidine-4-carboxylic acid | Orotic acid is a drug used to manage uncomplicated liver dysfunction in combination with xanthine. | 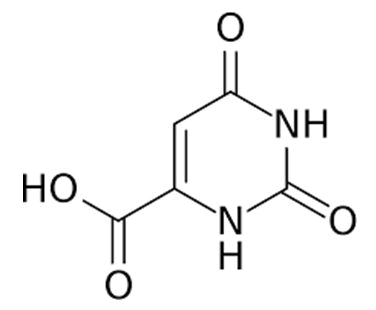 |
| Disorders associated with hyperuricaemia | |||
| Tisopurine | 1H-Pyrazolo[3,4-d]pyrimidine-4-thiol | Tisopurine (or thiopurinol) is a drug used in the treatment of gout in some countries. It reduces uric acid production through inhibiting an early stage in its production. | 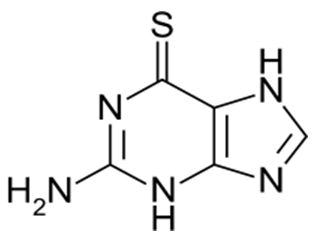 |
Table 1: List of pyrimidine and fused pyrimidine marketed drugs.
Medicinal significance
During the last two decades, several pyrimidine derivatives have been developed as chemotherapeutic agents and have found wide clinical applications.
Antineoplastics and anticancer agents: There are multiple antimetabolites with pyrimidine bases. They typically share structural similarities with the endogenous substrates they oppose. It's possible for the pyrimidine ring or the hanging sugar groups to undergo structural change [8]. One of the first metabolites made was the pyrimidine derivative 5-fluorouracil. Additionally, 5-thiouracil demonstrates some beneficial anti-cancer properties.
Drugs for hyperthyroidism: Thiobarbital, an alkyl derivative of 2-thiouracil, and both are efficient treatments for hyperthyroidism. With little side effects, propylthiouracil is prescribed as a medication for hyperthyroidism.
Antifolates, antibacterial and antiprotozoals: Significantly, Hitchings noted that several 2,4-diaminopyrimidines and some 2-amino-4-hydroxypyrimidines are folic acid antagonists. Since then, numerous 2,4-diaminopyrimidines have been created as antifolates in synthetic laboratories [9]. It was ultimately established that these pyrimidines block the Dihydrofolate Reductase Enzyme (DHFR) among the 2,4-diaminopyrimidine medications, pyrimethamine stands out as a specific inhibitor of malarial plasmodia's DHFR. A bacterial DHFR inhibitor that is both effective and selective, as opposed to the more potent but non-selective DHFR inhibitors methotrexate and aminopterin.
Sulfa drugs: Sulfadiazine, Sulfamerazine, and Sulfadimidine are pyrimidine derivatives of sulfa medicines that are superior to many other sulphonamides and are used to treat various acute UT infections, cerebrospinal meningitis, and people with penicillin allergies. Combinations of sulphonamide and trimethoprim are frequently used to treat opportunistic infections in AIDS patients.
Sulfadoxine, a sulfonamide with a half-life of 7-9 days, is used for malaria prevention. It is a short and intermediate acting sulfonamide. In veterinary medicine, sulfisomidine, which has a half-life of 7 hours, is used as part of a combined sulfa treatment. Sulfadiazine, sulfamerzine, and sulfadimidine are safe even for patients with impaired renal functions due to their good water solubility and low risk of kidney injury.
Antivirals and anti-AIDS: Pyrimidine derivatives have generated wide-spread interest due to their antiviral properties. 5-Iodode-oxyuridine is an antiviral agent of high selectivity.
Antibiotics: There are relatively few pyrimidines antibiotic examples. Bacimethrin (5-hydroxymethyl-2-methoxy-pyrimidin-4-amine), which is effective against many staphylococcal infections, is the most basic of all. Mycobacteria, along with a number of other gram-positive and gram-negative bacteria, are all susceptible to the cytosine derivative gourgetin [10]. Amicetin and plicacetin are two more cytosine derivatives that exhibit effectiveness against acid-fast and gram-positive bacteria as well as several other organisms. A broad range of antitrypanosomal action is seen in puromycin.
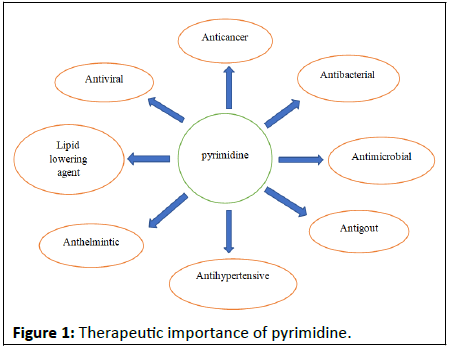
Antibiotics with aminoglycosides phleomycin, bleomycin, and related families are pyrimidine containing broad spectrum antibiotics. According to reports, the antitumor effects of another antibiotic, tubercidine, are present. They also exhibit antineoplastic action. Clinical trials with bleomycin against some tumours, including Hodgkin's lymphoma and diffuse testicular cancer, have already been conducted (Figure 1).
SAR of pyrimidine marketed drugs
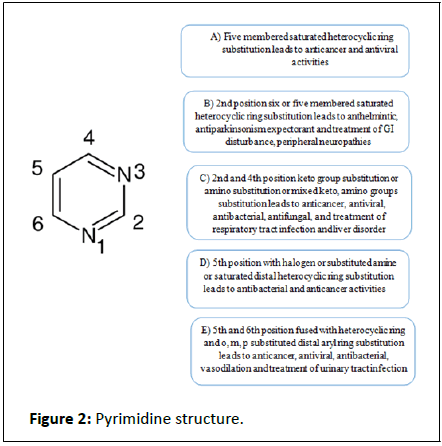
As SAR studies give insights into the molecular properties causing receptor affinity and selectivity. The promising nature of the compounds may be attributed to the substitutions at the hydrophobic domain. These compounds had electron withdrawing and donating groups at the ortho, meta and para position of the hydrophobic aryl ring. In general, it was observed that the substituted derivatives were more active than the other derivatives [11]. This may be because of the fact that the substituted derivatives are better fitted into the receptor site. In all of the pioneering experiments important core fragments is defined by presence of Hydrogen Donor/Acceptor unit (HAD), hydrophobic domain (A) (aryl ring substituted/unsubstituted) and electron donor atom (D). This common feature was found in the structures of well-established pyrimidine drugs (Figure 2).
Discussion
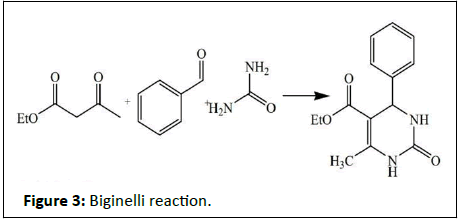
A pyrimidine has many properties in common with pyridine, as the number of nitrogen atoms in the ring increases the ring pi electrons become less energetic and electrophilic aromatic substitution gets more difficult while nucleophilic aromatic substitution gets easier. An example of the last reaction type is the displacement of the amino group in 2-aminopyrimidine by chlorine and its reverse. Reduction in resonance stabilization of pyrimidines may lead to addition and ring cleavage reactions rather than substitutions. One such manifestation is observed in the Dimroth rearrangement. Compared to pyridine, N-alkylation and N-oxidation is more difficult, and pyrimidines are also less basic: The pKa value for protonated pyrimidine is 1.23 compared to 5.30 for pyridine. Pyrimidines can also be prepared within the laboratory by organic synthesis. One method is the classic Biginelli reaction. The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones from ethyl acetoacetate, an aryl aldehyde (such as benzaldehyde) and urea (Figure 3).
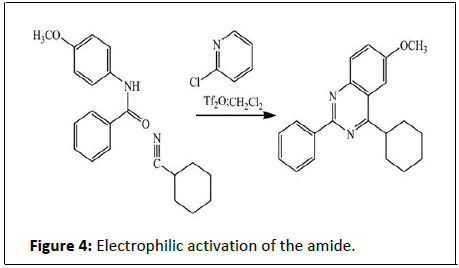
This reaction was developed by Pietro Biginelli in 1891. The reaction can be catalysed by Bronsted acids and/or by Lewis’s acids such as boron trifluoride. Several solid-phase protocols utilizing different linker combinations have been published. Dihydropyrimidinones, the products of the Biginelli reaction, are widely used in the pharmaceutical industry as calcium channel blockers, antihypertensive agents, and alpha-1-a-antagonists. Many other methods rely on condensation of carbonyls with amines for instance the synthesis of 2-Thio-6-methyluracil from thiourea and ethyl acetoacetate or the synthesis of 4-methylpyrimidine with 4,4-dimethoxy-2-butanone and formamide [12]. A novel method is by reaction of certain amides with carbonitriles under electrophilic activation of the amide with 2-chloro-pyridine and trifluoro methanesulfonic anhydride (Figure 4).
Antineoplastic, antiviral, antibacterial, expectorant, urinary tract infection, parkinsonism, anthelmintic, vasodilator, liver disorder, infections of the respiratory tract and ear, treatment of gastrointestinal roundworms, and pe have just a few of the many pharmaceutical and pharmacological uses for fused pyrimidine derivatives that have attracted significant interest in medicinal chemistry over the past several decades.
Conclusion
The biological functions of the pyrimidine scaffold have been described in this article. The pyrimidine's biological activities show its versatility and manoeuvrability, which offer medicinal chemists a continued interest in the pyrimidine skeleton in medicinal chemistry and drug development. The creation of effective and dependable techniques for the construction of these molecules will ensure that this is an active and significant area of research in heterocyclic chemistry. The anticancer potential of substituted pyrimidine at various positions, as well as SAR and chemical structure of novel anilino pyrimidine derivative pyrimidine fused with other heterocyclic ring, have all been covered in the current paper. The anticancer activity is greatly influenced by substitutions at the pyrimidine core's C-2, C-4, and C-6 positions, particularly a thio or amino group at C-2 and a modified phenyl group at C-4.
References
- Bajda M, Boryczka S, Malawsk B (2007) Investigation of lipophilicity of anticancer-active thioquinoline derivatives. Biomed Chromatogr 21:123-131
[Crossref] [Google Scholar] [PubMed]
- Bruno-Blanch L, Galvez J, Garcia-Domenac R (2003) Topological virtual screening: A way to find new anticonvulsant drugs from chemical diversity. Bioorg Med Chem Lett 13: 2749-2754
[Crossref] [Google Scholar] [PubMed]
- El-Baih FEM, Al-Rasheed HH, Hassan MA (2006) Microwave assisted synthesis of substitutefuran-2-carboxaldehydes and their reactions. J Saudi Chem Soc 9:575
- Estrada E, Pena, A (2000) In silico studies for the rational discovery of anticonvulsant compounds. Bioorg Med Chem 8:2755-2770
[Crossref] [Google Scholar] [PubMed]
- Mahapatra A, Prasad T, Sharma T (2021) Pyrimidine: A review on anticancer activity with key emphasis on SAR. Future J Pharm Sci 7:1-38
- Gore RP, Rajput AP (2013) A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Inven Today 5:148-152
- Martins MAP, Cunico W, Pereira CMP, Flores AFC, Bonacorso HG, et al. (2004) 4-Alkoxy-1,1,1-Trichloro-3-Alken-2-ones: Preparation and applications in heterocyclic synthesis. Curr Org Synth 1:391-403
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, et al. (2019) Cancer treatment and survivorship statistics. CA Cancer J Clin 69:363–385
[Crossref] [Google Scholar] [PubMed]
- MohanaRoopan S, Sompalle R (2016) Synthetic chemistry of pyrimidines and fused pyrimidines: A review. Synth Commun 46:645–672
- Prachayasittikul S, Pingaew R, Worachartcheewan A, Sinthupoom N, Prachayasittikul V, et al. (2017) Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev Med Chem 17:869–890
[Crossref] [Google Scholar] [PubMed]
- Dinakaran VS, Bomma B, Srinivasan KK (2012) Fused pyrimidines: The heterocycle of diverse biological and pharmacological significance. Der Pharma Chem 4:255-265
- Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55-63
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences